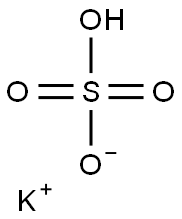
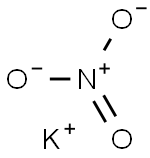
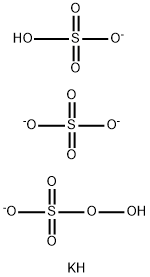
Potassium bisulfate
Synonym(s):Potassium bisulfate;Potassium hydrogen sulfate
- CAS NO.:7646-93-7
- Empirical Formula: HKO4S
- Molecular Weight: 136.16884
- MDL number: MFCD00011404
- EINECS: 231-594-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
What is Potassium bisulfate ?
Chemical properties
Potassium bisulfate, also known as potassium hydrogen sulfate and potassium acid sulfate, KHS04 is water soluble, white deliquescent crystals,which melt at 214°C. Potassium bisulfate dissolved in water undergoes ionization to form K+, H+, SO42-. It is used in wine making, fertilizer manufacture, and as a flux and food preservative.
The Uses of Potassium bisulfate
Potassium bisulfate may be used:
- As a catalyst for the preparation of butyl paraben from p-hydroxybenzoic acid and n-butyl alcohol.
- As a promoter for the synthesis of 3,4-dihydropyrimidin-2(1H)-one derivatives via Biginelli reaction in ethylene glycol.
- As an acid catalyst for the synthesis of ethyl tert-butyl ether (ETBE) from ethanol and tert-butyl alcohol via reactive distillation.
- As a dehydrating agent for the synthesis of pyruvic acid from tartaric acid.
The Uses of Potassium bisulfate
Potassium hydrogen sulfate is used in the conversion of tartrates to bitartrates in wine. It serves as a disintegrating agent in analytical chemistry and as an intermediate in the preparation of potassium persulfate by electrolysis. It is also employed in the manufacturing of fertilizers.
What are the applications of Application
Potassium bisulfate is the potassium salt of bisulfate anion used in the conversion of tartrates to bitartrates
What are the applications of Application
Potassium bisulfate may be used:
As a catalyst for the preparation of butyl paraben from p-hydroxybenzoic acid and n-butyl alcohol.
As a promoter for the synthesis of 3,4-dihydropyrimidin-2(1H)-one derivatives via Biginelli reaction in ethylene glycol.
As an acid catalyst for the synthesis of ethyl tert-butyl ether (ETBE) from ethanol and tert-butyl alcohol via reactive distillation.
As a dehydrating agent for the synthesis of pyruvic acid from tartaric acid.
Preparation
Potassium bisulfate is typically made by heating potassium sulfate (K2SO4) with sulfuric acid. The acid provides the hydrogen needed to convert the salt (K2SO4) to the corresponding acid salt (KHSO4).
General Description
Potassium hydrogen sulfate is a colorless crystalline solid with a sulfur odor. It may cause illness from ingestion. If heated to high temperatures it may emit toxic fumes. It is used as flux in analysis of ores and siliceous Compounds.
Air & Water Reactions
Fused salt is deliquescent, Soluble in water, yielding a corrosive acidic solution.
Reactivity Profile
Acidic salts, such as Potassium bisulfate , are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
Moderately toxic by ingestion. A corrosive irritant to the skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of SOx and K2O. Can form an explosive mixture. See also SULFATES.
Purification Methods
Crystallise it from H2O (1mL/g) between 100o and 0o. It is also formed when a warm solution of K2SO4 in conc H2SO4 is cooled down.
Properties of Potassium bisulfate
| Melting point: | 214 °C(lit.) |
| Boiling point: | decomposes [STR93] |
| Density | 2.32 g/mL at 25 °C(lit.) |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| appearance | Colorless solid |
| Specific Gravity | 2.322 |
| color | White |
| PH | 1 (50g/l, H2O, 20℃) |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,7613 |
| Stability: | Stable. Moisture sensitive. |
| CAS DataBase Reference | 7646-93-7(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium bisulfate (7646-93-7) |
Safety information for Potassium bisulfate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Potassium bisulfate
Potassium bisulfate manufacturer
JSK Chemicals
Buradon Inc.
Techno Pharmchem
ASM Organics
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


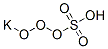
You may like
-
 Potassium hydrogen sulfate, fused CASView Details
Potassium hydrogen sulfate, fused CASView Details -
 Potassium hydrogen sulfate, fused CASView Details
Potassium hydrogen sulfate, fused CASView Details -
 Potassium Hydrogen Sulphate (Potassium Bisulphate) pure CAS 7646-93-7View Details
Potassium Hydrogen Sulphate (Potassium Bisulphate) pure CAS 7646-93-7View Details
7646-93-7 -
 Potassium hydrogen sulfate 99% CAS 7646-93-7View Details
Potassium hydrogen sulfate 99% CAS 7646-93-7View Details
7646-93-7 -
 Technical Grade Potassium Hydrogen Sulphate, 98%, 50Kg bagView Details
Technical Grade Potassium Hydrogen Sulphate, 98%, 50Kg bagView Details
7646-93-7 -
 Potassium BisulphateView Details
Potassium BisulphateView Details
7646-93-7 -
 Bio-Tech Grade Potassium Bisulphate Chemical, 98%, 50Kg bagView Details
Bio-Tech Grade Potassium Bisulphate Chemical, 98%, 50Kg bagView Details
7646-93-7 -
 POTASSIUM BI SULPHATE, 98%, 25Kg bagView Details
POTASSIUM BI SULPHATE, 98%, 25Kg bagView Details
7646-93-7
