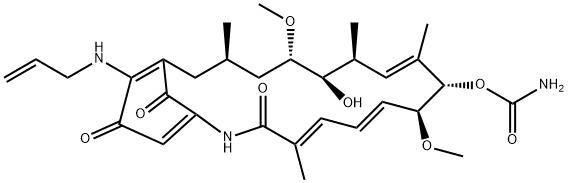
17-AAG
Synonym(s):17-(Allylamino)geldanamycin;17-AAG;17-Demethoxy-17-allylamino geldanamycin;Geldanamycin,17-demethoxy-17-(2-propenylamino)-;Tanespimycin
- CAS NO.:75747-14-7
- Empirical Formula: C31H43N3O8
- Molecular Weight: 585.69
- MDL number: MFCD04973892
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
What is 17-AAG?
Description
17-AAG (75747-14-7) is a semi-synthetic analog of geldanamycin (Cat.# 10-1084) which is less toxic and more stable. 17-AAG selectively binds to and inhibits HSP90 from tumor cells. Anti-angiogenic activity. Cell permeable.
Chemical properties
Dark Purple Solid
The Uses of 17-AAG
Potent inhibitor of heat shock protein 90 (Hsp90). 17-AAG is a less toxic analog than Geldanamycin. It induces apoptosis and displays antitumor effects. 17-AAG inhibits the activity of oncogenic proteins such as N-ras, Ki-ras, c-Akt, and p185erB2.
The Uses of 17-AAG
Geldanamycin is a potent inhibitor of Hsp90 that exhibits severe hepatotoxicity when used in vivo. 17-AAG is an analog of geldanamycin which has potent in vivo activity and reduced toxicity. Like other Hsp90 inhibitors, 17-AAG has diverse anti-tumor actions and has potential in treating certain types of cancer. 17-AAG inhibits the growth of prostate cancer cell lines (IC50 = 25-45 nM). 17-AAG promotes the degradation of HER2 and induces growth arrest and apoptosis in breast cancer cells overexpressing HER2 (IC50 = 4-72 nM).
The Uses of 17-AAG
17-(Allylamino)-17-demethoxygeldanamycin (17-AAG) inhibits heat shock protein 90 (Hsp90), the expression of heat shock factor-1, and the activity of oncogenic proteins such as N-ras, Ki-ras, c-Akt, and p185erB2. In addition to its anti-tumor effects, 17-AAG also induces apoptosis.
What are the applications of Application
17-AAG is a potent heat shot protein 90 Hsp90) inhibitor for BT474 tumor cells, derived from geldanamycin.
Definition
ChEBI: A 19-membered macrocyle that is geldanamycin in which the methoxy substituent attached to the benzoquinone moiety has been replaced by an allylamino group. It is a potent inhibitor of heat shock protein 90 (Hsp90). A less toxic analogue than geldanamycin, t induces apoptosis and displays antitumour effects.
General Description
Chemical structure: benzenoid
Biological Activity
Inhibitor of heat shock protein 90 (Hsp90) chaperone activity, and an analog of geldanamycin (9,13-Dihydroxy-8,14,19-trimethoxy-4,10,12,16-tetramethyl-2-azabicyclo[16.3.1]docosa-4,6,10,18,21-pentaene-3,20,22-trione, 9-carbamate ). Subsequently inhibits the activity of oncogenic proteins such as p185 erbB-2 (IC 50 = 31 nM), N-ras, Ki-ras and c-Akt. Antitumor in vivo .
Biochem/physiol Actions
17-(Allylamino)-17-demethoxygeldanamycin (17-AAG) is a benzoquinone and is an analog of geldanamycin.
Storage
-20°C
References
1) Schulte et al. (1998), The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin; Cancer Chemother. Pharmacol. 42 273 2) Kamal et al. (2003), A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors; Nature, (b>425 407 3) Kaur et al. (2004), Therapeutic and diagnostic implications of Hsp90 activation; Clinical Cancer Res. 10 4813
Properties of 17-AAG
| Melting point: | 201-203°C |
| Boiling point: | 797.8±60.0 °C(Predicted) |
| Density | 1.21 |
| RTECS | LX8932000 |
| Flash point: | >110°(230°F) |
| storage temp. | Keep in dark place,Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO: soluble |
| form | solid |
| pka | 8.62±0.70(Predicted) |
| color | Purple or dark red |
| Water Solubility | Soluble in DMSO at 150 mg/mL; soluble in ethanol at 5 mg/mL. Very poorly soluble in water |
| Sensitive | Light Sensitive |
| Stability: | Stable for 1 year from date of purchase as supplied. Protect from moisture. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 75747-14-7 |
Safety information for 17-AAG
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 17-AAG
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 17-(Allylamino)-17-demethoxygeldanamycin CAS 75747-14-7View Details
17-(Allylamino)-17-demethoxygeldanamycin CAS 75747-14-7View Details
75747-14-7 -
 Tanespimycin 98.00% CAS 75747-14-7View Details
Tanespimycin 98.00% CAS 75747-14-7View Details
75747-14-7 -
 17-AAG CAS 75747-14-7View Details
17-AAG CAS 75747-14-7View Details
75747-14-7 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8