Phoxim
- CAS NO.:14816-18-3
- Empirical Formula: C12H15N2O3PS
- Molecular Weight: 298.3
- MDL number: MFCD05863734
- EINECS: 238-887-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-28 17:34:51
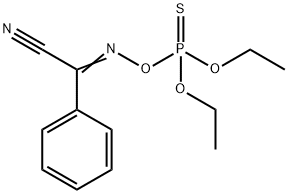
What is Phoxim?
Description
Phoxim is a light yellow liquid, Solubility in water is 1.5 mg/L (20 ?C). It is readily soluble in most organic solvents and slightly in aliphatic hydrocarbons. Log Kow = 3.38. It is relatively slowly hydrolyzed in aqueous media; DT50 (22 ?C) at pH 4, 7, and 9 is 26.7, 7.2, and 3.1 d, respectively.
Chemical properties
yellowish oily liquid, Specific gravity: 1.176 (20°C)
The Uses of Phoxim
Phoxim is used to control stored product insect pests in silos and barns. It is also used to control household insects such as ants and other public health pests. Agricultural targets include caterpillars and soil insects in maize, vegetables, cotton and potatoes.
The Uses of Phoxim
Insecticide.
Safety Profile
Poison by ingestion. An experimental teratogen. When heated to decomposition it emits very toxic fumes of CN-, NO,, PO,, and SO,. See also NITRILES.
Metabolic pathway
The primary metabolic step in the metabolism of phoxim in most biological systems is hydrolysis to the oxime, which may occur either oxidatively or via oxidative desulfuration followed by hydrolysis of the oxon which is much more hydrolytically labile. Stage II metabolism in plants involves the oxime group of the oxaminophenylacetonitrile moiety being reduced to an amine followed by its conjugation with malonic acid. Phoxim is one of a quite large group of insecticidal organophosphates which generates tetraethyl pyrophosphate and its thio analogues under the influence of light. These are compounds which are very active as acetylcholinesterase inhibitors and highly toxic to mammals.
Metabolism
Phoxim is oxidatively desulfurated to the oxon, which inhibits housefly AChE 270 times as quickly as bovine AChE. In mammals, the oxon is immediately hydrolyzed into diethyl hydrogen phosphate. The direct cleavage of the oxime ester bond of phoxim, the hydrolytic transformation of the nitrile group into carboxyl, and deethylation also contribute to the low mammalian toxicity. Elimination is very quick, 97% of the dose being excreted in the urine and feces in 24 h. Degradation in soils is also very rapid. By photochemical reactions, the thiooxime phosphate isomer, tetraethyl pyrophosphate, and its monothio analog are produced in small amounts on foliage.
Degradation
Phoxim is relatively slowly hydrolysed in water. The half-lives at pH 4,7
and 9 were 26.7, 7.2 and 3.1 days, respectively (PM). Drager (1977)
reported a half-life of 145 min in pH 11.5 50% aqueous propanol at 37 °C.
It is gradually decomposed under UV irradiation (PM).
The photodecomposition of [32P]phoximon cotton leaves and glass
plates was investigated by Drager (1971) who characterised the more
hydrophobic organic-soluble components by TLC, GLC and 1H NMR and
MS of the purified products. The cotton plants were irradiated with
natural sunlight and mercury-vapour lamps at an average light intensity
of 3500-4000 lux. On both glass plates and irradiated cotton leaves,
phoxim was degraded relatively quickly to one major hydrophobic component,
the concentration of which reached a maximum after about one
day. This material was identified as the thiono-thiolo rearranged compound
O,O-diethyl a-cyanobenzylideneamino-thiophosphorothioate (2).
This transformation is an activation step as 2 is a highly active acetylcholinesterase
inhibitor. No metabolite 2 was formed on cotton leaves
placed in the dark so it can be considered to be a photochemical product
rather than a plant metabolite. Additional minor metabolites were tetraethyl
pyrophosphate (3) and O,O,O',O'-tetraethyl thiophosphorothioate
(4) which formed to a greater extent on the glass plates rather than the
cotton leaves.
Toxicity evaluation
The acute oral LD50 for rats is >2000 mg/kg. Inhalation LC50 (4 h) for rats is >4.0 mg/L air. NOEL (2 yr) for rats is 15 mg/kg diet (0.75 mg/kg/d). ADI is 1 μg/kg b.w.
Properties of Phoxim
| Melting point: | 5.55°C |
| Boiling point: | bp0.01 102° |
| Density | d420 1.176 |
| vapor pressure | 2.1×10-3 Pa (20 °C) |
| refractive index | nD20 1.5405 |
| storage temp. | 0-6°C |
| solubility | Acetonitrile (Slightly), Chloroform (Slightly), Methanol (Slightly) |
| form | liquid |
| Water Solubility | 1.5 mg l-1 (20 °C) |
| color | Colorless to light yellow |
| Stability: | Hygroscopic, Moisture Sensitive |
| CAS DataBase Reference | 14816-18-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Phoxim(14816-18-3) |
| EPA Substance Registry System | Phoxim (14816-18-3) |
Safety information for Phoxim
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H311:Acute toxicity,dermal H317:Sensitisation, Skin H330:Acute toxicity,inhalation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for Phoxim
| InChIKey | ATROHALUCMTWTB-WYMLVPIESA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
