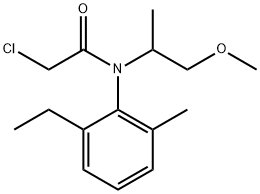
Metolachlor
Synonym(s):2-Chloro-N-(2-ethyl-6-methylphenyl)-N-(-2-methoxy-1-methylethyl)acetamide
- CAS NO.:51218-45-2
- Empirical Formula: C15H22ClNO2
- Molecular Weight: 283.79
- MDL number: MFCD00055293
- EINECS: 257-060-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
What is Metolachlor?
Description
Metolachlor is a colorless or tan to brown,oily liquid with a slightly sweet odor. Molecularweight 5 283.83; Boiling point = 100℃ at 0.001 mmHg. Itis stable to about 300℃. Hazard Identification (based onNFPA-704 M Rating System): Health 1, Flammability 0,Reactivity 0. Slightly soluble in water.
Chemical properties
Pale Yellow Oil
Chemical properties
Metolachlor is a colorless or tan to brown, oily liquid. Slightly sweet odor.
The Uses of Metolachlor
A selective pre-emergence herbicide.
The Uses of Metolachlor
Preemergence herbicide used to control most annual grasses and many annual weeds in beans, chickpeas, corn, cotton, milo, okra, peanuts, peas, potatoes, safflower, soybeans and some ornamentals.
Definition
ChEBI: 2-chloro-N-(2-ethyl-6-methylphenyl)-N-(1-methoxypropan-2-yl)acetamide is an organochlorine compound that is 2-chloroacetamide substituted by a (2-ethyl-6-methylphenyl)-N-(1-methoxypropan-2-yl) group at the nitrogen atom. It is an aromatic amide, an ether, a member of benzenes and an organochlorine compound.
General Description
Tan to brown oily liquid with a slightly sweet odor. Slightly soluble in water and denser than water. Hence sinks in water. Soluble in most organic solvents. Used as a selective herbicide.
Air & Water Reactions
Slightly soluble in water. Hydrolyzed by strong mineral acids and strong alkalis.
Reactivity Profile
Metolachlor is a chlorinated acetamide. May react with azo and diazo compounds to generate toxic gases. May form flammable gases with strong reducing agents. Combustion generates mixed oxides of nitrogen (NOx).
Health Hazard
Can cause skin irritation and eye irritation. Headache and nausea may occur following prolong exposure.
Agricultural Uses
Herbicide: Not approved for use in EU countries. Registered for use in the U.S. Metolachlor is a selective herbicide that is usually applied to crops before plants emerge from the soil, and is used to control certain broadleaf and annual grassy weeds in field corn, soybeans, peanuts, grain sorghum, potatoes, pod crops, cotton, safflower, stone fruits, nut trees, highway rights-of-way and woody ornamentals. Prior to the RED of April, 1995, its primary use was on corn, soybeans and sorghum. It inhibits protein synthesis; thus, high-protein crops (e. g., soy) can be adversely affected by excessive metolachlor application. Additives may be included in product formulations to help protect sensitive crops (i.e., sorghum) from injury.
Trade name
BICEP®[C]; BROADSTRIKE®; CGA- 24705®; CINCH®; CODAL®; COTORAN® MULTI®; CYCLE®[C] DREXEL ME-TOO-LACHLOR®; DUAL®; DUAL MAGNUM®; DUET®[C]; INTER PLUS®; MEDAL®[C]; MILOCEP®; ONTRACK 8E®[C]; PENNANT®[C]; PRELUDE®[C]; PRIMAGRAM®; PRIMEXTRA®; TURBO®
Potential Exposure
Metolachlor is a chlorinated acetamide selective herbicide used for weed control in corn and for controlling grasses in a variety of crops including cotton and peanuts.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Note to physician: Treat for methemoglobinemia.Spectrophotometry may be required for precise determination of levels of methemoglobin in urine.
Environmental Fate
Soil. Metolachlor and its degradation products combine with humic acids in soils and
small quantities are degraded to carbon dioxide (Ashton and Monaco, 1991). In soil, the
fungus Chaetomium globosum degraded metolachlor to 2-chloro-N-(2-ethyl-6-methylphenyl)
acetamide, 2-hydroxy-N-(2-methylvinylphenyl)-N-(methoxyprop-2-yl)acetamide, 3-
hydroxy-8-methyl-N-(methoxyprop-2-yl)-2-oxo-1,2,3,4-tetrahydraquinoline, 2-chloro-N-
(2-ethyl-6-methylphenyl)-N-(hydroxyprop-2-yl)acetamide and the tentatively identified
compounds 3-hydroxy-1-isopropyl-8-methyl-2-oxo-1,2,3,4-tetrahydroquinoline, N-(methoxyprop-
2-yl)-8-methyl-2-oxo-1,2,3,4-tetrahydroquinoline, N-(methoxyprop-2-yl)-N-(2-
methyl-6-vinyl)aniline and 1-(methoxyprop-2-yl)-7-methyl-2,3-dihydroindole (McGahen
8). Metolachlor was transformed by a strain of soil actinomycetes to the following products:
2-chloro-N-(2-ethyl-6-methylphenyl)-N-(hydroxyprop-2-yl)acetamide, 2-chloro-N-
2-(1-hydroxyethyl)-6-(methylphenyl)-N-(hydroxyprop-2-yl)acetamide, 2-chloro-N-(2-
ethyl-6-hydroxymethylphenyl)-N-(hydroxy-prop-2-yl)-acetamide, diastereoisomers of 2-
chloro-N-(2-ethyl-6-hydroxymethylphenyl)-N-(methoxyprop-2-yl)acetamide and 2-
chloro-N-2-(hydroxyethyl)-6-hydroxymethylphenyl)-N-(methoxyprop-2-yl)acetamide.
These products were formed via hydroxylation of both the N-alkyl and alkyl side chains
(Krause et al., 1985). In sterilized soil, metolachlor did not degrade after 4 months
(Bouchard et al., 1982).
Zimdahl and Clark (1982) reported half-lives of 15–38 and 33–100 days for the
herbicide in clay loam soil and sandy loam soil, respectively. They also reported that soil
moisture increased the dissipation rate. At 20°C, the dissipation rates of metolachlor in
the clay loam and sandy loam soils at 20, 50 and 80% soil moisture contents were 0.028,
0.053, 0.062 and 0.016, 0.028 and 0.037/day, respectively. The half-lives of metolachlor
in soil at maintained at temperatures of 30 and 40°C were approximately 3.85 and 2.75
weeks, respectively (Bravermann et al., 1986). The reported half-lives of metolachlor in
soil is approximately 6 days (Worthing and Hance, 1991) and 3–4 weeks (Bowman, 1988).
Groundwater. According to the U.S. EPA (1986) metolachlor has a high potential to
leach to groundwater.
Plant. Metabolizes in plants forming water soluble, polar, nonvolatile products (Hartley
and Kidd, 1987) and glutathione conjugates (Breaux et al., 1987).
Chemical/Physical. The volatilization half-life of metolachlor in an unstirred solution
was 20 days at 40°C. Volatilization is not significant at temperatures < 25°C (Lau et al.,
1995).
Metabolic pathway
A stable bacterial community absorbs and transforms metolachlor from a liquid medium. From the medium of the 7 day-old culture of the bacterial community, 2- hydroxy-N-(2-methyl-6-vinylphenyl)-N-(2-methoxy- methylethyl)acetamide and 4-(2-ethyl-6-methylphenyl)- 5-methyl-3-morpholinone are identified. The products recovered from cells of J4-A include dechlorinated metolachlor, a thiol compound, a more complicated conjugate, and a non-sulfur-containing conjugate. By sorghum microsomes, O-demethylation occurs in the metolachlor degradation process.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from oxidizers. Where possible, automatically pump liquid from drums or other storage containers to process containers
Shipping
UN2902 Pesticides, liquid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitrates. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic, and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur).
Waste Disposal
In accordance with 40CFR 165 recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of Metolachlor
| Melting point: | 25°C |
| Boiling point: | bp0.001 100° |
| Density | 1.1200 |
| refractive index | 1.526-1.529 |
| Flash point: | 2 °C |
| storage temp. | APPROX 4°C
|
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Liquid |
| pka | 1.45±0.50(Predicted) |
| form | neat |
| color | Colorless to light yellow |
| Odor | wh. to tan clear liq., odorless |
| Water Solubility | 529.7mg/L(20 ºC) |
| BRN | 8396147 |
| CAS DataBase Reference | 51218-45-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Metolachlor(51218-45-2) |
| EPA Substance Registry System | Metolachlor (51218-45-2) |
Safety information for Metolachlor
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Metolachlor
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Metolachlor 98% (GC) CAS 51218-45-2View Details
Metolachlor 98% (GC) CAS 51218-45-2View Details
51218-45-2 -
 Metolachlor CAS 51218-45-2View Details
Metolachlor CAS 51218-45-2View Details
51218-45-2 -
 Metolachlor solution CAS 51218-45-2View Details
Metolachlor solution CAS 51218-45-2View Details
51218-45-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1