Phosphorus tribromide
Synonym(s):Phosphorus tribromide;Phosphorus(III) bromide
- CAS NO.:7789-60-8
- Empirical Formula: Br3P
- Molecular Weight: 270.69
- MDL number: MFCD00011436
- EINECS: 232-178-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
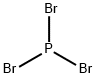
What is Phosphorus tribromide?
Chemical properties
Phosphorus tribromide is a colourless liquid(possibly hazy) with the formula PBr3. It fumes in air due to hydrolysis and has a penetrating odour. It is widely used in the laboratory for the conversion of alcohols toalkyl bromides.
Phosphorus tribromide (PBr3) is commonly used in Hell-Volhard-Zelinsky halogenation for the α-bromination of carboxylic acids to form the corresponding acyl bromide. It is also useful for the conversion of primary and secondary alcohols to alkyl bromides.
The Uses of Phosphorus tribromide
Phosphorus tribromide is used mainly for the conversion of alcohols to alkyl bromides and it is used in tests for skin corrosivity. It is used for alkene preparation and it act as a lewis base and a lewis acid. Phosphorus tribromide is used in the manufacture of pharmaceuticals such as alprazolam, methohexital and fenoprofen.
Phosphorus tribromide may be used as a reagent:
In a novel protocol for synthesizing 7-ethoxy-1-(p-ethylphenoxy)-3,7-dimethyl-2-octene, a synthetic juvenile hormone mimic of trans,trans,cis-10,11-epoxy-7-ethyl-3,11-dimethyltrideca-2,6-dienoate.
To synthesize 1,2,3-diazaphosphinane, 1,2,3-thiazaphosphinine and 1,2-azaphosphole bearing a chromone ring.
To synthesize 1-Bromoundec-1-ene from 10-undecen-1-ol.
The Uses of Phosphorus tribromide

To a solution of the SM (1.12 g, 6.66 mmol) in dry ether (19 mL) at 0 C was added dropwise PBr3 (0.626 mL, 6.66 mmol). The ice bath was removed and the reaction was stirred for 3 h. H2O was carefully added to the mixture and the layers were separated. The org layer was washed with brine, dried (MgSO4), and concentrated in vacuo to provide the product. [1.49 g, 97%]
The Uses of Phosphorus tribromide
Phosphorus(III) bromide is used mainly for the conversion of alcohols to alkyl bromides and it is used in tests for skin corrosivity. It is used for alkene preparation and it act as a lewis base and a lewis acid. It is used as a catalyst for the alpha-bromination of carboxylic acids and used as intermediates in hell-volhard-zelinsky halogenation. Phosphorus tribromide is used in the manufacture of pharmaceuticals such as alprazolam, methohexital and fenoprofen. It is also used as a fire suppression agent.
What are the applications of Application
Phosphorus Tribromide is a component to test for skin corrosivity.
Preparation
Phosphorus tribromide, PBr3, is most conveniently prepared by reaction between liquid bromine and a solution of white phosphorus in PBr3. In most of its reactions, the tribromide resembles the trichloride although the former has been much less studied and in some cases the products seem to be more complex.
Reactions
Silyl phosphites such as (EtO)2POSiMe3 and (Et3SiO)3P are known. The latter compound can be prepared in 30% yield by reacting phosphorus tribromide with an organosilane in the presence of zinc chloride. Esters of the former type can be made by reaction.
3Et3SiO+PBr3–-(Et3SiO)3+3RBr
Et3SiONa+(EtO)2PCl–-(EtO)2POSiEt3+NaCl)
General Description
A colorless fuming liquid with a pungent odor. Corrosive to metals and tissue. Boiling point 347°F (175°C). Freezing point -40°F (-40°C).
Air & Water Reactions
Fumes in air. Decomposed by water to form phosphoric acid and hydrobromic acid. Reaction with warm water is very rapid and may be violent [Mellor v.8. 1032 1940].
Reactivity Profile
Phosphorus tribromide reacts with oxidizing agents to generate heat and products that may be flammable, combustible, or otherwise reactive; the reactions may be violent. Forms complexes with potassium or sodium metal that explode when shocked. Drop wise addition to 3-phenylpropanol caused an explosion when stirring of the mixture was discontinued [Chem. Brit., 1974, 10, 101-102].
Health Hazard
Inhalation causes severe irritation of nose, throat, and lungs. Ingestion causes burns of mouth and stomach. Contact with eyes or skin causes severe burns.
Safety Profile
Probably highly toxic. A corrosive irritant to the eyes, skin, and mucous membranes. Wdl react with water, steam, or acids to produce heat, toxic and corrosive fumes. Violent reaction or ignition with calcium hydroxide + sodium carbonate, phenylpropanol, sulfuric acid, oleum, fluorosulfuric acid, chlorosulfuric acid, 1,1,1 -tris(hydroxymethyl)methane, water, potassium, sodium, RuO4. When heated to decomposition it emits very toxic fumes of Brand POx. See also PHOSPHIDES and BROMIDES.
Purification Methods
It is decomposed by moisture, it should be kept dry and is corrosive. Purify it by distillation through an efficient fractionating column [see Whitmore & Lux J Am Chem Soc 54 3451] in a slow stream of dry N2, i.e. under strictly dry conditions. [Gay & Maxson Inorg Synth II 147 1946, Org Synth Col Vol II 358 1943.] Dissolve it in CCl4, dry it over CaCl2, filter and distil it. Store it in sealed ampoules under N2 and keep it away from light. HARMFUL VAPOURS.
Properties of Phosphorus tribromide
| Melting point: | -41.5 °C (lit.) |
| Boiling point: | 175 °C (lit.) |
| Density | 2.88 g/mL at 20 °C (lit.) |
| vapor density | 9.3 (vs air) |
| vapor pressure | 0.27 psi ( 54 °C) |
| refractive index | n |
| Flash point: | 172.9°C |
| storage temp. | Store below +30°C. |
| solubility | soluble in Ether,Acetone,Chloroform |
| appearance | Colorless liquid |
| form | Liquid |
| color | Clear to slightly hazy colorless to yellow or light brown |
| Specific Gravity | 2.88 |
| Odor | Irritating odour |
| Water Solubility | reacts |
| FreezingPoint | -40℃ |
| Sensitive | Moisture Sensitive |
| Merck | 14,7357 |
| BRN | 3903060 |
| Dielectric constant | 3.9(20℃) |
| Stability: | Stable, but reacts violently with water. Incompatible with moisture, strong bases, strong oxidizing agents, reactive metals, acids, alcohols. |
| CAS DataBase Reference | 7789-60-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Phosphorus tribromide(7789-60-8) |
| EPA Substance Registry System | Phosphorus tribromide (7789-60-8) |
Safety information for Phosphorus tribromide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Phosphorus tribromide
| InChIKey | IPNPIHIZVLFAFP-UHFFFAOYSA-N |
Phosphorus tribromide manufacturer
M/s Anji Biosciences
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Phosphorus(III) Bromide CAS 7789-60-8View Details
Phosphorus(III) Bromide CAS 7789-60-8View Details
7789-60-8 -
 Phosphorus(III) bromide CAS 7789-60-8View Details
Phosphorus(III) bromide CAS 7789-60-8View Details
7789-60-8 -
 Phosphorous Tribromide ChemicalView Details
Phosphorous Tribromide ChemicalView Details
7789-60-8 -
 PHOSPHORUS TRIBROMIDE, Grade: ArView Details
PHOSPHORUS TRIBROMIDE, Grade: ArView Details
7789-60-8 -
 PHOSPHOROUS TRIBROMIDEView Details
PHOSPHOROUS TRIBROMIDEView Details
7789-60-8 -
 Phosphorus TribromideView Details
Phosphorus TribromideView Details
7789-60-8 -
 Phosphorous TribromideView Details
Phosphorous TribromideView Details
7789-60-8 -
 Phosphorus TribromideView Details
Phosphorus TribromideView Details
7789-60-8
