Iodotrimethylsilane
Synonym(s):TMIS;Trimethyliodosilane
- CAS NO.:16029-98-4
- Empirical Formula: C3H9ISi
- Molecular Weight: 200.09
- MDL number: MFCD00001028
- EINECS: 240-171-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
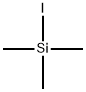
What is Iodotrimethylsilane?
Chemical properties
Straw liquid
The Uses of Iodotrimethylsilane
Iodotrimethylsilane is used for the introduction of trimethylsilyl group in organic synthesis. It is also useful for gas chromatography analysis by converting alcohol into a silyl ether derivative, thereby making it more volatile than the original molecule.
The Uses of Iodotrimethylsilane

To a solution of the SM (0.16 g, 0.39 mmol) in CHCl3 (3.0 mL) was added dropwise TMSI (93 mg, 0.46 mmol) at RT. The reaction mixture was stirred at RT overnight. The mixture was concentrated in vacuo and the resulting residue was dissolved in H2O (10 mL) and acidified with 1N HCl to pH ~3. The aq layer was extracted with EtOAc (3 x 30 mL). The aq layer was then basified with 5% aq Na2CO3 to pH ~9, and was extracted with 3:1 CHCl3/IPA (3 x 30 mL). The combined organics were washed with brine (3 x 30 mL), dried (Na2SO4), and concentrated to provide the product as a brown syrup. [100 mg, 83%]
What are the applications of Application
Iodotrimethylsilane is An efficient reagent for ether, ester, carbamate, ketal, and lactone cleavage.
Preparation
Iodotrimethylsilane is prepared by mixing equimolar amounts of chlorotrimethylsilane and sodium iodide in acetonitrile, reduces various benzylic alcohols to the corresponding phenylalkanes. Other have been reported for the preparation of Trimethylsilyl Iodide(TMS-I): chlorotrimethylsilane undergoes halogen exchange with either lithium iodide in CHCl3 or sodium iodide in MeCN, which allows in situ reagent formation. Alternatively, hexamethyldisilane reacts with iodine at 25–61°C to afford TMS-I with no byproducts.
What are the applications of Application
Iodotrimethylsilane can be used as a versatile reagent for the mild dealkylation of ethers, carboxylic esters, lactones, carbamates, acetals, phosphonate and phosphate esters; cleavage of epoxides, cyclopropyl ketones; conversion of vinyl phosphates to vinyl iodides; neutral nucleophilic reagent for halogen exchange reactions, carbonyl and conjugate addition reactions; use as a trimethylsilylating agent for formation of enol ethers, silyl imino esters, and N-silylenamines, alkyl, alkenyl and alkynyl silanes; Lewis acid catalyst for acetal formation, α- alkoxymethylation of ketones, for reactions of acetals with silyl enol ethers and allylsilanes; reducing agent for epoxides, enediones, α-ketols, sulfoxides, and sulfonyl halides; dehydrating agent for oximes.
Reactions
Trimethylsilyl iodide reacts under mild conditions in the absence of a catalyst with alkyl fluorides as well as with benzyl and tertiary alkyl chlorides and bromides to give good yields of alkyl iodides and the corresponding trimethylsilyl halides.
General Description
Iodotrimethylsilane is a multipurpose reagent used in various organic reactions. It is used for the dealkylation of few compounds like lactones, ethers, acetals, and carbamates and trimethylsilylating agent for the synthesis of silyl imino esters, alkyl and alkenyl silanes, etc. It also acts as a Lewis acid catalyst and as a reducing agent in many organic reactions.
Synthesis
This procedure describes a convenient method for the preparation of Iodotrimethylsilane: A 250-ml., two-necked, round-bottomed flask equipped with a magnetic stirring bar, an addition funnel for solids, and a reflux condenser bearing a nitrogen inlet is charged with 5.6 g. (0.21 mole) of aluminum powder and 16.2 g. (0.100 mole) of hexamethyldisiloxane and purged with nitrogen. The mixture is stirred and heated with an oil bath at 60°C as 50.8 g. (0.200 mole) of iodine is added slowly through the addition funnel over 55 minutes. The bath temperature is raised to ca. 140°C, and the mixture is heated under reflux for 1.5 hours. The reflux condenser is removed, and the flask is equipped for distillation at atmospheric pressure. The bath temperature is gradually raised from 140°C to 210°C, and the clear, colorless distillate is collected, yielding 32.6–35.3 g. (82–88%) of iodotrimethylsilane, b.p. 106–109°C.
Purification Methods
Add a little antimony powder and fractionate with this powder in the still. 20 1.470. Stabilise the distillate with 1% wt of Cu powder. [Eaborn J Chem Soc 3077 1950, Beilstein 4 IV 4009.]
Properties of Iodotrimethylsilane
| Melting point: | <0°C |
| Boiling point: | 106 °C(lit.) |
| Density | 1.406 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | −25 °F |
| storage temp. | -20°C |
| solubility | reacts |
| form | Liquid |
| appearance | Colorless liquid |
| Specific Gravity | 1.47 |
| color | Clear colorless to reddish |
| Water Solubility | reacts |
| Sensitive | Moisture & Light Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 1731136 |
| Stability: | Moisture Sensitive (Reactive) |
| CAS DataBase Reference | 16029-98-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Iodotrimethylsilane(16029-98-4) |
| EPA Substance Registry System | Silane, iodotrimethyl- (16029-98-4) |
Safety information for Iodotrimethylsilane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H225:Flammable liquids H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Iodotrimethylsilane
| InChIKey | CSRZQMIRAZTJOY-UHFFFAOYSA-N |
Iodotrimethylsilane manufacturer
JSK Chemicals
ASM Organics
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran


You may like
-
 Trimethylsilyl Iodide 98%View Details
Trimethylsilyl Iodide 98%View Details
16029-98-4 -
 Iodotrimethylsilane 16029-98-4 98%View Details
Iodotrimethylsilane 16029-98-4 98%View Details
16029-98-4 -
 Iodotrimethylsilane CAS 16029-98-4View Details
Iodotrimethylsilane CAS 16029-98-4View Details
16029-98-4 -
 Trimethylsilyl Iodide (stabilized with Aluminum) CAS 16029-98-4View Details
Trimethylsilyl Iodide (stabilized with Aluminum) CAS 16029-98-4View Details
16029-98-4 -
 Trimethylsilyl iodide 16029-98-4 98%View Details
Trimethylsilyl iodide 16029-98-4 98%View Details
16029-98-4 -
 Iodotrimethylsilane, 97% 99%View Details
Iodotrimethylsilane, 97% 99%View Details
16029-98-4 -
 Iodotrimethylsilane CAS 16029-98-4View Details
Iodotrimethylsilane CAS 16029-98-4View Details
16029-98-4 -
 Iodotrimethylsilane CAS 16029-98-4View Details
Iodotrimethylsilane CAS 16029-98-4View Details
16029-98-4
