Phenyl dichlorophosphate
Synonym(s):Phenyl phosphodichloridate;Phenyl phosphorodichloridate;Phenyl phosphoryl dichloride
- CAS NO.:770-12-7
- Empirical Formula: C6H5Cl2O2P
- Molecular Weight: 210.98
- MDL number: MFCD00002067
- EINECS: 212-220-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
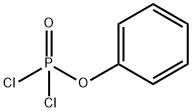
What is Phenyl dichlorophosphate?
Chemical properties
clear colourless to very slightly brown liquid
The Uses of Phenyl dichlorophosphate
In organic synthesis for preparation of lactams, phosphate diesters and for oxidation reactions.
The Uses of Phenyl dichlorophosphate
Reagent for preparation of phosphate diesters. Phenyl dichlorophosphate is used in the preparation of symmetrical phosphate diesters. It is used as a phosphorylating agent for alcohols and amines. It is also involved in the preparation of phenylphosphoro di-(1-imidazolidate) and 2-phenyl-bis-triazoloylphosphate, which is used in peptide synthesis. It plays a vital role in the Pfitzner-Moffatt oxidation which involves oxidation of alcohols to the corresponding aldehydes and ketones. Furthermore, it is utilized in Beckmann rearrangement for the preparation of N-phenylacetamide from acetophenone oxime.
Preparation
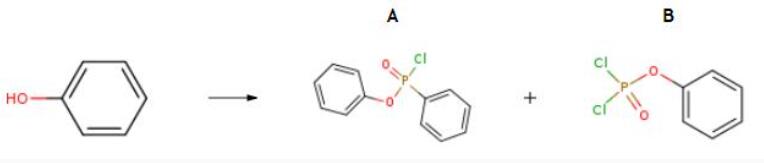
The preparation of Phenyl dichlorophosphate is as follows:160 g (1 mol) of phosphorus oxychloride and 1 g of titanium tetrachloride were respectively added to a four-necked flask to carry out the reaction, and the temperature was raised to 70-80° C., and phenol (94 g, 1 mol) was slowly added dropwise. The phenol addition time was about 2- 3 hours,After completion of the dropwise addition, the reaction is maintained at 80-90° C. for 3-5 hours. After no hydrogen chloride is evolved, the reaction is completed. After the reaction, the phosphorus oxychloride is distilled under reduced pressure, the vacuum degree is 30-100 KPa, and the distillation temperature is 30-100° C. Distillation under reduced pressure to obtain the intermediate of the mixture, a mono-substituted and di-substituted mixture of phosphorus oxychloride, the ratio of the mixture is 5:5, gas phase analysis to obtain the intermediate of the mixture, the yield of the intermediate is 95%, phosphorus oxychloride The recovery rate is 95% (calculated as phosphorus oxychloride).
General Description
A liquid. May severely irritate skin, eyes and mucous membranes.
Air & Water Reactions
About the same density as water and moderately soluble in water.
Reactivity Profile
An hologenated organophosphate. Organophosphates are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.
Hazard
May cause severe skin, eye, and mucous membrane irritation; Reacts violently with water; Causes burns; Inhalation may cause corrosive injuries to upper respiratory tract and lungs; Toxic by ingestion; Highly toxic by skin absorption; Dermal LD50 (guinea pig) = 14-400 mg/kg.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Properties of Phenyl dichlorophosphate
| Melting point: | -1 °C |
| Boiling point: | 241-243 °C (lit.) |
| Density | 1.412 g/mL at 25 °C (lit.) |
| vapor pressure | 2.33Pa at 25℃ |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | soluble in Chloroform |
| form | Liquid |
| color | Clear colorless to very slightly brown |
| Water Solubility | Decomposes |
| Sensitive | Moisture Sensitive |
| Merck | 14,7282 |
| BRN | 511863 |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 770-12-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Phosphorodichloridic acid, phenyl ester(770-12-7) |
| EPA Substance Registry System | Phosphorodichloridic acid, phenyl ester (770-12-7) |
Safety information for Phenyl dichlorophosphate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Phenyl dichlorophosphate
| InChIKey | TXFOLHZMICYNRM-UHFFFAOYSA-N |
Phenyl dichlorophosphate manufacturer
Adishank Chemicals Pvt Ltd
SOLFYN INTERNATIONAL LLP
Horizon Chemicals
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 770-12-7 99%View Details
770-12-7 99%View Details
770-12-7 -
 O-phenyl-phosphorodichloridate 98%View Details
O-phenyl-phosphorodichloridate 98%View Details -
 Phenyl dichlorophosphate, ≥95% CAS 770-12-7View Details
Phenyl dichlorophosphate, ≥95% CAS 770-12-7View Details
770-12-7 -
 Phenyl Dichlorophosphate CAS 770-12-7View Details
Phenyl Dichlorophosphate CAS 770-12-7View Details
770-12-7 -
 770-12-7 PHENYL DICHLOROPHOSPHATE 99%View Details
770-12-7 PHENYL DICHLOROPHOSPHATE 99%View Details
770-12-7 -
 770-12-7 O-phenyl-phosphorodichloridate 98%View Details
770-12-7 O-phenyl-phosphorodichloridate 98%View Details
770-12-7 -
 Phenyl phosphorodichloridate 99% CAS 770-12-7View Details
Phenyl phosphorodichloridate 99% CAS 770-12-7View Details
770-12-7 -
 Phenyl dichlorophosphate CAS 770-12-7View Details
Phenyl dichlorophosphate CAS 770-12-7View Details
770-12-7