PENITREM A
Synonym(s):Tremortin A
- CAS NO.:12627-35-9
- Empirical Formula: C37H44ClNO6
- Molecular Weight: 634.2
- MDL number: MFCD12547934
- EINECS: 634-132-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 16:48:35
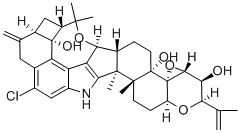
What is PENITREM A?
Description
Penitrem A (12627-35-9) is a fungal mycotoxin that acts as a selective, irreversible blocker of the smooth muscle high conductance Ca2+-activated K+?(BK, KCa1.1) channel (100% block at 10 nM).1?Displays brain neurotoxicity in rats along with dose-dependent convulsions and death.2?An important tool for studying the role of BK channels in vascular function which is effective in cellular, tissue and?in vivo?experiments.3?Inhibits BK channels in inside-out and cell-attached patches, whereas iberiotoxin (considered the gold standard BK channel blocker) does not.3?May be used to partially ablate Purkinje cells in immature rat cerebellum providing a model for neural stem cell transplantation studies.4
Chemical properties
Solid
Occurrence
A series of closely related metabolites of Penicillium crustosum has recently been isolated and the components separated chromatographically. Penitrem A forms colourless crystals from EtOH.
The Uses of PENITREM A
Penitrem A is a tremorgenic mycotoxin isolated from Penicillium species. Penitrem A is a selective blocker of high-conductance Ca2+-activated potassium channels.
The Uses of PENITREM A
Penitrem A has been used as Ca2+-Activated K+ (BK) channel blocker to study its effect on BK current in bag cell neurons.
What are the applications of Application
Penitrem A is a selective and irreversible blocker of the smooth muscle high conductance Ca2+-activated K+ channel
Definition
ChEBI: Penitrem A is an organooxygen compound and an organic heterotricyclic compound.
Biochem/physiol Actions
Penitrem A intoxication causes ataxia, polypnea, and sustained tremors and may lead to seizures and death. Penitrem A affects the central and peripheral nervous system. It impairs the GABAergic neurotransmission in the cerebellum.
Safety Profile
Poison by ingestion and intraperitoneal routes. An experimental teratogen. When heated to decomposition it emits very toxic fumes of Clí and NOx.
storage
-20°C
References
References/Citations:
Properties of PENITREM A
| Melting point: | 238°C |
| Density | 1.0241 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (up to 6 mg/ml) |
| pka | 13.22±0.70(Predicted) |
| form | White solid. |
| color | Amorphous solid |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
Safety information for PENITREM A
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral |
Computed Descriptors for PENITREM A
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Penitrem A CAS 12627-35-9View Details
Penitrem A CAS 12627-35-9View Details
12627-35-9 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1