PEMIROLAST
- CAS NO.:69372-19-6
- Empirical Formula: C10H8N6O
- Molecular Weight: 228.21
- MDL number: MFCD00864611
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
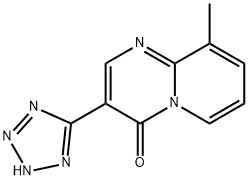
What is PEMIROLAST?
Originator
Alamast, Santen
The Uses of PEMIROLAST
Anti-allergic; inhibitor (mediator release).
Indications
For the prevention of itching of the eyes caused by allergies such as hay fever, and allergic conjunctivitis
Background
Pemirolast potassium is a slightly yellow powder that is soluble in water. It is a mast cell stabilizer that acts as an antiallergic agent. As an ophthalmic aqueous sterile solution, pemirolast is used for the prevention of itching of the eyes caused by allergies such as hay fever, and allergic conjunctivitis. Pemirolast is potentially useful for prophylaxis of pulmonary hypersensitivity reactions to drugs such as paclitaxel.
Definition
ChEBI: Pemirolast is a pyridopyrimidine.
Manufacturing Process
Ferrous nitrate hexahydrate (60 mg) followed by sodium (4.5 g, 0.196 gatom) were added to liquid ammonia. To this mixture was added a solution of 3-methylpyridine (10.0 g, 0.093 mole) in N,N-dimethylaniline (21 ml) over a period of 5 min. The ammonia was allowed to evaporate and the residue heated under nitrogen by means of an oil bath maintained at 180°C for 18 h. The cooled residue was treated with ice (50 g) followed by 2 N sodium hydroxide (50 ml). The mixture was triturated for 2 h and then filtered. The collected solid was washed with boiling toluene (2 times 100 ml). The toluene layer was separated from the combined filtrate and washings, concentrated to about 50 ml and extracted with 5% aqueous acetic acid (5 times 20 ml). The combined extracts were filtered and reduced to dryness. The residue was recrystallized from methylcyclohexane to give 2-amino-3-methylpyridine acetate (4.9 g, 29%), melting point 85°-95°C. The acetate (2.5 g, 1.37 mmoles) was briefly suspended in 1 N sodium hydroxide (50 ml). The mixture was extracted with methylene chloride. The extract was washed with water, dried, and concentrated to give 2-amino-3-methylpyridine as an oil.
A solution of 2-amino-3-methylpyridine (5.0 g, 0.0462 mole) and ethyl ethoxymethylenecyanoacetate (7.82 g, 0.0462 mole) in toluene (4 ml) was heated for 15 min by means of an oil bath maintained at 100°C. The solution was cooled and the crude product (9.1 g, 85%) collected by filtration. The product was recrystallized from 2-propanol to give an analytical sample of ethyl 2-cyano-3-(3-methyl-2-pyridylamino)acrylate, melting point 144°146°C.
Aluminum chloride (3.51 g, 0.0263 mole) was added to cold (-30°C) tetrahydrofuran (180 ml). Sodium azide (5.12 g, 0.0788 mole) was added and the mixture heated under reflux for 30 min. The mixture was cooled to 5°C. Ethyl 2-cyano-3-(3-methyl-2-pyridylamino)acrylate (5.0 g, 0.0216 mole) was added and the mixture heated under reflux for 18 h. The tetrahydrofuran was removed under reduced pressure. The residue was treated with ice water (100 ml) and acidified to pH 3 with 6 N hydrochloric acid. The mixture was filtered and the collected solid recrystallized from N,N-dimethylformamide to give the 9-methyl-3-(1H-tetrazol-5-yl)-4H-pyrido[1,2-a]pyrimidin-4-one (2.5 g, 50.7%). Melting point 310°-311°C, dec.
Potassium hydroxide was added dropwise to a stirred mixture of 9-methyl-3(1H-tetrazol-5-yl)-4H-pyrido[1,2-a]pyrimidin-4-one in water .The mixture was diluted with water to a volume of about 300 ml and was then heated to a temperature of 50°C during 2 min. The mixture was filtered and the water removed from the filtrate by lyophilization. The residue was recrystallized from water:ethanol to give the 9-methyl-3-(1H-tetrazol-5-yl)-4H-pyrido[1,2
2632 a]pyrimidin-4-one potassium salt
brand name
Alamast (Sanofi Winthrop).
Therapeutic Function
Antiallergic, Antiulcer
Pharmacokinetics
Pemirolast is used for the prophylactic treatment of itching of the eye associated with allergic conjunctivitis. Pemirolast potassium is a mast cell stabilizer that inhibits the in vivo Type I immediate hypersensitivity reaction. Pemirolast inhibits the antigen-induced release of inflammatory mediators (e.g., histamine, leukotriene C4, D4, E4) from human mast cells. Allergic reactions lead to cell-degranulation and the release of histamine (and other chemical mediators) from the mast cell or basophil. Once released, histamine can react with local or widespread tissues through histamine receptors. Histamine, acting on H1-receptors, produces pruritis and vasodilatation (allowing blood fluids to enter the area to cause swelling). Pemirolast is a histamine H1 antagonist. It competes with histamine for the normal H1-receptor sites on effector cells of blood vessels to provide effective, temporary relief of watery and itchy eyes.
Clinical Use
Pemirolast, with an acidic tetrazole isosteric replacement for a carboxylic acid functionality, is used topically in the eye to prevent itching associated with allergic conjunctivitis. It is an inhibitor of the release of histamine and other inflammatory mediators, including leukotrienes. Significant use as a systemic agent has been reported, and it has been shown to be of value in preventing restenosis after percutaneous coronary angiopathy.
Metabolism
Hepatic (Ophthalmic)
Properties of PEMIROLAST
| Melting point: | 310-311° (dec) |
| Boiling point: | 454.8±55.0 °C(Predicted) |
| Density | 1?+-.0.1 g/cm3(Predicted) |
| pka | 4.00±0.50(Predicted) |
| CAS DataBase Reference | 69372-19-6(CAS DataBase Reference) |
Safety information for PEMIROLAST
Computed Descriptors for PEMIROLAST
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1