Orlistat
Synonym(s):(−)-Tetrahydrolipstatin;N-Formyl-L-leucine (1S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl ester;Orlistat;Ro-18-0647
- CAS NO.:96829-58-2
- Empirical Formula: C29H53NO5
- Molecular Weight: 495.73
- MDL number: MFCD05662360
- EINECS: 639-755-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
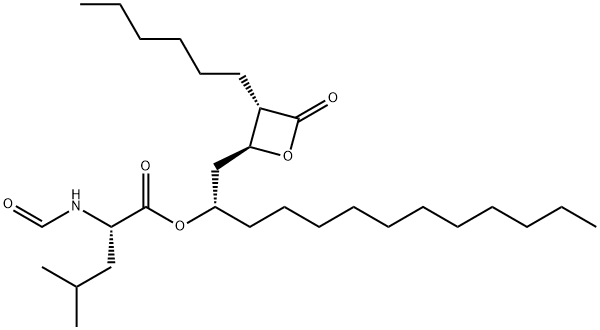
What is Orlistat?
Absorption
The systemic absorption and exposure of orlistat is low, however, systemic absorption of the drug is not required for orlistat activity. After an oral dose with 360 mg of radiolabeled orlistat, plasma radioactivity achieved a peak at about 8 hours. Plasma concentrations of unchanged parent drug were close to the lower end of detection limits (<5 ng/mL). In plasma samples of patients taking orlistat, the detection of unchanged drug was sporadic and very low concentrations were detected (<10 ng/mL or 0.02 μM) with no evidence suggesting drug accumulation.
Toxicity
The oral LD50 of orlistat is >5000 mg/kg in rats. Single orlistat doses of 800 mg and multiple doses of up to 400 mg three times a day for 15 days have been administered to healthy weight and obese subjects without clinically significant adverse findings. In addition, doses of 240 mg three times a day have been given to obese patients for 6 months without a significant adverse effects. Post-marketing reports of overdoses cases indicate no adverse events or adverse events that are similar to those reported with the recommended dose. If a significant overdose with orlistat occurs, the patient should be observed for at least 24 hours. Based on the results of clinical studies, systemic effects caused by orlistat are likely to be rapidly reversible.
Description
Orlistat is an obesity drug sold under the trade names Xenical (Roche) and Alli (GlaxoSmithKline). It helps prevent the absorption of fat in the gut, but it also causes gastrointestinal side effects. Orlistat was recently used in an attempt to accelerate the elimination of a dioxin derivative from a poisoning victim.
Description
Orlistat was launched in the UK as Xenical for the long-term treatment of obesity, preferably in conjunction with a moderately reduced calorie diet. Orlistat is a tetrahydro-derivative of the natural hypolipaemic lipstatin (from Streptomyces toxyfncini) and can be obtained either by hydrogenation of lipstatine or by several different synthetic ways involving many steps from ( S ) - rnalic acid. Orlistat is a potent inhibitor of gastrointestinal lipases required for the lipolysis and digestion of dietary fat, in particular of pancreatic lipase ; as a result, it prevents the absorption of about one third of the fat contained in food and acts as an effective weight-reducing therapy. The outcomes of several clinical trials involving thousands of obese patients showed that Orlistat promotes a significant weight loss (often between 5 and 10% after one year) and improves cardiovascular risk factors such as total cholesterol, LDL/HDL ratio, blood glucose levels, insulin, blood pressure. Orlistat has minimal systemic absorption, the majority of the compound itself being recovered in the feces ; it does not affect other gastrointestinal processes, or the absorption of other rnacronutrients such as carbohydrates and proteins.
Description
Orlistat is a digestive lipase inhibitor. It inhibits diacylglycerol lipase α (DAGLα), DAGLβ, α/β-hydrolase domain-containing protein 12 (ABHD12), ABHD16A, and platelet-activating factor acetylhydrolase (PAF-AH; IC50s = 0.06, 0.1, 0.08, 0.03, and 0.05 μM, respectively), as well as pancreatic lipase and hormone-sensitive lipase (IC50s = 0.65 and 2.1 μg/ml, respectively) but does not inhibit fatty acid amide hydrolase (FAAH) or KIAA1363 (IC50s = >100 μM for both). Orlistat decreases ionomycin-induced production of the endocannabinoid 2-arachidonoyl glycerol (2-AG) in N18TG2 murine neuroblastoma cells when used at a concentration of 1 μM. It also inhibits fatty acid synthase (FASN; Kiapp = ~0.1 μM for the human enzyme) and the proliferation of PC3 prostate cancer cells in a concentration-dependent manner. Orlistat (10 mg/kg) decreases serum cholesterol levels and total body weight in a mouse model of obesity induced by a high-fat diet. Formulations containing orlistat have been used in the treatment of adult obesity.
Chemical properties
Off-White Solid
Originator
Roche (Switzerland)
The Uses of Orlistat
Orlistat is an antiobesity agent. Orlistat is an pancreatic lipase inhibitor.
Lipase Inhibitor, THL is an inhibitor of pancreatic and gastric lipase, and an activator of caspase-3.
The Uses of Orlistat
An antiobesity agent. A pancreatic lipase inhibitor. Antiobesity agent.
The Uses of Orlistat
antidiabetic
The Uses of Orlistat
Tetrahydrolipstatin (orlistat) is a semi-synthetic derivative of lipstatin, a metabolite isolated from Streptomyces toxytricini. Tetrahydrolipstatin acts as a potent, irreversible inhibitor of pancreatic lipase. In vivo, it blocks the absorption of triglycerides while allowing fatty acid absorption. Tetrahydrolipstatin is widely used for the treatment of obesity.
Background
The global prevalence of obesity is on a rapid incline, leading to a multitude of complications that impose a significant personal and economic burden. These complications diminish the quality of life and escalate healthcare costs. While diet and exercise are foundational for weight management, they may prove insufficient for some individuals. In such cases, pharmacological or surgical intervention becomes necessary. Orlistat, a lipase inhibitor, stands as one such pharmacological intervention employed in the treatment of obesity. Functioning by inhibiting fat-metabolizing enzymes, it was granted FDA approval in 1999 for use in conjunction with a reduced-calorie diet. Orlistat has established itself as a generally well-tolerated and effective weight-loss aid. Presently, it is accessible in both over-the-counter and prescription formulations, the choice between which depends on the prescribed dosage quantity.
Indications
Orlistat is indicated for obesity management including weight loss and weight maintenance when used in combination with calorie reduction in overweight and obese adults; this indication applies to both the prescription formulation of 120 mg and the over-the-counter formulation of 60 mg. Orlistat in the 120 mg prescription formulation is also indicated to reduce the risk of weight regain following weight loss.
Definition
ChEBI: Orlistat is a carboxylic ester resulting from the formal condensation of the carboxy group of N-formyl-L-leucine with the hydroxy group of (3S,4S)-3-hexyl-4-[(2S)-2-hydroxytridecyl]oxetan-2-one. A pancreatic lipase inhibitor, it is used as an anti-obesity drug. It has a role as an EC 3.1.1.3 (triacylglycerol lipase) inhibitor, a bacterial metabolite, an EC 2.3.1.85 (fatty acid synthase) inhibitor and an anti-obesity agent. It is a beta-lactone, a L-leucine derivative, a member of formamides and a carboxylic ester.
brand name
Xenical (Roche).
General Description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Orlistat is a specific lipase inhibitor derived from lipostatin, which is naturally produced by Streptomyces toxytricini. Lipase inhibition induced by orlistat reduces the absorption of dietary fat, thereby contributing to caloric deficit.
Biological Activity
Hypolipemic pancreatic, gastric and carboxylester lipase inhibitor. Exhibits no activity at phospholipase A 2 , liver esterase, trypsin and chymotrypsin. Inhibits the thioesterase domain of fatty acid synthase, leading to cell cycle arrest at the G 1 /S boundary in vitro . Prevents the absorption of approximately one third of fat from food and exhibits progastrokinetic, antiobesity and antihypercholesterolemic activity in vivo .
Biochem/physiol Actions
Orlistat, used in obesity research, is a pancreatic lipase inhibitor that acts locally in the gastrointestinal tract to inhibit lipase.
Pharmacokinetics
Orlistat helps with weight reduction and maintenance by inhibiting the absorption of dietary fats via the inhibition of lipase enzymes.
Clinical Use
Adjunct in obesity
Drug interactions
Potentially hazardous interactions with other drugs
Acarbose: avoid concomitant administration.
Amiodarone: possibly slightly reduces absorption.1
Anticoagulants: monitor INR more frequently (due
to reduction in vitamin K absorption).1
Antiepileptics: possible increased risk of convulsions.
Antivirals: absorption of abacavir, atazanavir,
darunavir, didanosine, efavirenz, elvitegravir,
emtricitabine, enfuvirtide, etravirine, fosamprenavir,
indinavir, lamivudine, lopinavir, maraviroc,
nevirapine, raltegravir, rilpivirine, ritonavir,
saquinavir, stavudine, tenofovir, tipranavir and
zidovudine possibly reduced.
Ciclosporin: possibly reduces absorption of
ciclosporin.
Tacrolimus: possibly reduces absorption of
tacrolimus.1
Thyroid hormones: possible increased risk of
hypothyroidism with levothyroxine.
Vitamins: may reduce the absorption of fat soluble
vitamins.
Metabolism
Orlistat is hydrolyzed in the intestinal wall. In a radiolabeled orlistat mass balance study in obese patients, two metabolites were identified. The first metabolite, M1, was the hydrolyzed β-lactone ring product of orlistat. The second metabolite, M3, was produced from M1’s cleavage of the N-formyl leucine side-chain. Both metabolites accounted for about 42% of total plasma radioactivity. Both M1 and M3 are considered pharmacologically inactive.
Metabolism
Orlistat is minimally absorbed and has no defined
systemic pharmacokinetics. The metabolism of orlistat
occurs mainly within the gastrointestinal wall to form 2
major inactive metabolites, M1 (4-member lactone ring
hydrolysed) and M3 (M1 with N-formyl leucine moiety
cleaved).
Faecal excretion of the unabsorbed drug is the major route
of elimination. Approximately 97% of the administered
dose is excreted in faeces and 83% of that as unchanged
orlistat.
Storage
+4°C
References
1) Bisogno et al. (2006), Development of the first potent and specific inhibitors of endocannabinoid biosynthesis; Biochim. Biophys. Acta, 1761 205 2) Hadvary et al. (1991), The lipase inhibitor tetrahydrolipstatin bind covalently to the putative active site serine of pancreatic lipase; J. Biol. Chem., 266 2021 3) Kridel et al. (2004), Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity; Cancer Res., 64 2070 4) Ballinger and Peikin (2002), Orlistat: it’s current status as an anti-obesity drug; Eur. J. Pharmacol., 440 109
Properties of Orlistat
| Melting point: | <50 °C |
| Boiling point: | 615.9±30.0 °C(Predicted) |
| alpha | D20 -32.0° (c = 1 in chloroform) |
| Density | 0.976±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: 19 mg/mL |
| pka | 14.59±0.23(Predicted) |
| form | solid |
| color | white |
| Merck | 14,6869 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 week. |
| CAS DataBase Reference | 96829-58-2(CAS DataBase Reference) |
Safety information for Orlistat
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Orlistat
| InChIKey | AHLBNYSZXLDEJQ-FWEHEUNISA-N |
| SMILES | C(O[C@H](C[C@H]1[C@H](CCCCCC)C(=O)O1)CCCCCCCCCCC)(=O)[C@H](CC(C)C)NC=O |
Orlistat manufacturer
Kavya Pharma
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Orlistat 99%View Details
Orlistat 99%View Details -
 Orlistat, ≥98% CAS 96829-58-2View Details
Orlistat, ≥98% CAS 96829-58-2View Details
96829-58-2 -
 Orlistat 95% CAS 96829-58-2View Details
Orlistat 95% CAS 96829-58-2View Details
96829-58-2 -
 Orlistat 98.00% CAS 96829-58-2View Details
Orlistat 98.00% CAS 96829-58-2View Details
96829-58-2 -
 Orlistat API PowderView Details
Orlistat API PowderView Details
96829-58-2 -
 Orlistat CAS NO : 96829-58-2, Grade Standard: IPView Details
Orlistat CAS NO : 96829-58-2, Grade Standard: IPView Details
96829-58-2 -
 Orlistat api Powder Raw MaterialView Details
Orlistat api Powder Raw MaterialView Details
96829-58-2 -
 Orlistat PowderView Details
Orlistat PowderView Details
96829-58-2
