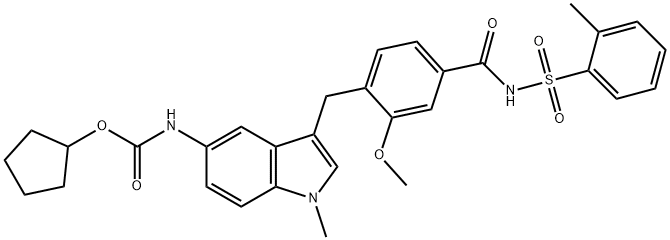
Zafirlukast
Synonym(s):N-[3-[[2-Methoxy-4-[[[(2-methylphenyl)sulfonyl]amino]carbonyl]phenyl]methyl]-1-methyl-1H-indol-5-yl]carbamic acid cyclopentyl ester
- CAS NO.:107753-78-6
- Empirical Formula: C31H33N3O6S
- Molecular Weight: 575.68
- MDL number: MFCD00864775
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
What is Zafirlukast?
Absorption
Rapidly absorbed following oral administration, reduced following a high-fat or high-protein meal.
Toxicity
Side effects include rash and upset stomach.
Description
Accolate was launched in Ireland, Finland and the US for treatment of asthma. Prepared via an eight step synthesis from methyl 3-methoxy-4- methylbenzoate, zafirlukast acts as a LTD4 antagonist and is the first compound of a new class of drugs. LTC4, LTD4 and LTE4 were determined to be the constituents of the slow-reacting substance of anaphylaxis (SRS-A) which was found to induce asthma effects (bronchoconstriction, increased vascular permeability resulting in edema, cellular infiltration of airway tissues and decreased mucociliary transport). Thus an inhibitor of their synthesis would at least attenuate these symptoms. Zafirlukast binds to the CysLT receptor LT-1 and blocks the effect of LTC4, LTD4 and LTE4. The drug is an oral twice daily formulation that reversed an LTD4 challenge, attenuated the response of platelet-activating factor (PAF), an allergen and cold air challenge and exercise-induced asthma.
Chemical properties
Off-white to pale pink crystalline solid
Originator
Zeneca (UK)
The Uses of Zafirlukast
Zafirlukast has been used to stimulate pancreatic β cell line (MIN6) and pancreatic islets for insulin secretion assay. It may be used as an adenosine triphosphate-binding cassette transporter (ABCG2) inhibitor in MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] cytotoxicity assay in human embryonic kidney cells (HEK293) and in Kirby-Bauer disc diffusion assays, bactericidal activity and minimal inhibitory concentration (mIC) assay against M. smegmatis
The Uses of Zafirlukast
A potent, selective and orally active CysLT receptor antagonist. Leukotriene D4 antagonist. Used as an antiasthmatic
The Uses of Zafirlukast
antiasthmatic;leukotriene receptor antagonist (LTRA)
Background
Zafirlukast is an oral leukotriene receptor antagonist (LTRA) for the maintenance treatment of asthma, often used in conjunction with an inhaled steroid and/or long-acting bronchodilator. It is available as a tablet and is usually dosed twice daily. Another leukotriene receptor antagonist is montelukast (Singulair), which is usually taken just once daily.
Zafirlukast blocks the action of the cysteinyl leukotrienes on the CysLT1 receptors, thus reducing constriction of the airways, build-up of mucus in the lungs and inflammation of the breathing passages.
Indications
For the prophylaxis and chronic treatment of asthma.
What are the applications of Application
Zafirlukast is a potent and selective CysLT1 receptor antagonist
Definition
ChEBI: Zafirlukast is a member of indoles, a carbamate ester and a N-sulfonylcarboxamide. It has a role as an anti-asthmatic agent and a leukotriene antagonist.
Manufacturing Process
6-Nitroindol with 4-bromobenzyl-3-methoxy-benzoic acid methyl ester gives in presence of silver oxide catalyst the diarilmethane 2-(2,4-dimethoxy-benzyl)- 5-nitro-1H-indole. The indole nitrogen is then converted to its anion with sodium hydride; treatment with methyl iodide gives the corresponding N- methyl derivative. Catalitic hydrogenation then converts the nitro group to the amine to give 4-(6-amino-1H-inden-2-ylmethyl)-3-methoxy-benzoic acid methyl ester. Acylation of that amine with cyclophentylchloroformate then gives the urethane [2-(2,4-dimethoxy-benzyl)-1-methyl-1H-indol-5-yl]- carbamic acid cyclopentyl ester. The benzoate ester is then selectively cleaved with lithium hydroxide in dimethyl formamide to the corresponding carboxylic acid. The intermediate is converted to the acyl sulfonamide by coupling with ortho-toluenesulfonamide to give [2-(2-methoxy-4-o- tolylmethanesulfonylaminocarbonyl-benzyl)-1-methyl-1H-indol-5-yl]-carbamic acid cyclopentyl ester (zafirlucast).
brand name
Accolate (AstraZeneca).
Therapeutic Function
Anti-asthmatic
Acquired resistance
Zafirlukast inhibits CYP3A4 and CYP2C9 in concentrations equivalent to clinical plasma levels and, therefore, should be used with caution in patients taking drugs metabolized by these enzymes. Specifically, coadministration with warfarin results in a significant increase in prothrombin time. Other drugs metabolized by CYP2C9 are phenytoin and carbamazepine. In addition, CYP3A4-metabolized drugs are cyclosporine, cisapride, and the dihydropyridine class of calcium channel blockers. Of particular interest is the fact that aspirin increases the plasma levels of zafirlukast, and theophylline decreases the plasma levels of zafirlukast. Care should be taken when coadministering with erythromycin, because this decreases the bioavailability of zafirlukast.
Biochem/physiol Actions
Zafirlukast plays a key role in alleviating mucus and airway constriction in asthma based inflammatory response. It regulates pancreatic β cells for the secretion of insulin and this functionality is interlinked to the calcium based phosphorylation of the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and protein kinase B (AKT). Zafirlukast has inhibitory potential on ATP-binding cassette (ABC) transporters and reverses the multidrug resistance function of ATP-binding cassette super-family G member 2 (ABCG2). Zafirlukast inhibits mycobacterial nucleoid-associated protein Lsr2 and halts the growth of Mycobacteria.
Pharmacokinetics
Zafirlukast is a synthetic, selective peptide leukotriene receptor antagonist (LTRA) indicated for the prophylaxis and chronic treatment of asthma. Patients with asthma were found in one study to be 25-100 times more sensitive to the bronchoconstricting activity of inhaled LTD4 than nonasthmatic subjects. In vitro studies demonstrated that zafirlukast antagonized the contractile activity of three leukotrienes (LTC4, LTD4 and LTE4) in conducting airway smooth muscle from laboratory animals and humans. Zafirlukast prevented intradermal LTD4-induced increases in cutaneous vascular permeability and inhibited inhaled LTD4-induced influx of eosinophils into animal lungs.
Clinical Use
Zafirlukast is an indole derivative with a sulfonamide group that fulfills the need for an ionizable moiety on the pharmacophore. A large number of analogues have been prepared; however, they all resulted in a decrease in antagonist activity. Zafirlukast, like montelukast, is a selective antagonist for the cysLT1 receptor and antagonizes the bronchoconstrictive effects of all leukotrienes (LTC4, LTD4, and LTE4).
Veterinary Drugs and Treatments
While zafirlukast potentially could be useful in treating feline asthma, including allowing dose reductions of corticosteroid therapy, its efficacy has been disappointing to this point and most do not recommend its use. Potentially, it could be of benefit in allergymediated (where leukotrienes play a role) dermatologic conditions, such as atopy in dogs, but evidence has been that it is not very effective.
Drug interactions
Potentially hazardous interactions with other drugs
Aminophylline: possibly increases aminophylline
concentration; zafirlukast concentration reduced.
Analgesics: concentration increased by aspirin.
Antibacterials: concentration reduced by
erythromycin.
Anticoagulants: may enhance the effects of warfarin.
Theophylline: possibly increases theophylline
concentration; zafirlukast concentration reduced.
Metabolism
Hepatic
Metabolism
Zafirlukast is well absorbed orally; however, food will decrease its absorption by as much as 40%. Zafirlukast is primarily metabolized in the liver by CYP2C9 and CYP3A4 to hydroxylated metabolites. Zafirlukast also has been shown to undergo carbamate hydrolysis, followed by N-acetylation. Additionally, zafirlukast in known to produce an idiosyncratic hepatotoxicity in susceptible patients. This is appears to result from the formation of an electrophilic α,β-unsaturated iminium intermediate evidenced by the formation of a glutathione adduct on the methylene carbon bridging the indole ring to the methoxybenzene moiety of the molecule. More than 90% if its metabolites are excreted in the feces, with the remaining found in the urine.
References
1) Adkins and Brogden, (1998), Zafirlukast. A review of its pharmacology and therapeutic potential in the management of asthma; Drugs, 55 121 2) Woszczek et al., (2010), Concentration-dependent noncysteinyl leukotriene type 1 receptor-mediated inhibitory activity of leukotriene receptor antagonists; J. Immunol. 184 2218 3) Wyttenbach & Kuentz (2017), Glass-forming ability of compounds in marketed amorphous drug products; Eur. J. Pharm. and Biopharm., 112 204 [Focus Citation]
Properties of Zafirlukast
| Melting point: | 139°C |
| Density | 1.32±0.1 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: ≥28mg/mL |
| form | solid |
| pka | 4.94±0.10(Predicted) |
| color | White or pink |
| Merck | 14,10108 |
| Stability: | Stable for 2 years as supplied. Protect from exposure to moisture. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 107753-78-6(CAS DataBase Reference) |
| EPA Substance Registry System | Carbamic acid, N-[3-[[2-methoxy-4-[[[(2-methylphenyl)sulfonyl]amino]carbonyl]phenyl]methyl]-1-methyl-1H-indol-5-yl]-, cyclopentyl ester (107753-78-6) |
Safety information for Zafirlukast
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Zafirlukast
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![10,11-Dihydro-alpha-methyl-10-oxo-dibenzo[b,f]thiepin-2-acetic acid](https://img.chemicalbook.in/CAS/GIF/74711-43-6.gif)
![4-[(5-Amino-1-methyl-1H-indol-3-yl)methyl]-3-methoxy-N-[(2-methylphenyl)sulfonyl]benzamide](https://img.chemicalbook.in/CAS2/GIF/219583-10-5.gif)
![Cyclopentyl 3-[2-Methoxy-4-(p-tolylsulfonylcarbaMoyl)benzyl]-1-Methylindol-5-ylcarbaMate](https://img.chemicalbook.in/CAS/GIF/1159195-70-6.gif)
You may like
-
 107753-78-6 ZafirlukastStandard IHS 97%View Details
107753-78-6 ZafirlukastStandard IHS 97%View Details
107753-78-6 -
 107753-78-6 98%View Details
107753-78-6 98%View Details
107753-78-6 -
 Zafirlukast 98%View Details
Zafirlukast 98%View Details
107753-78-6 -
 Zafirlukast 99%View Details
Zafirlukast 99%View Details -
 Zafirlukast CAS 107753-78-6View Details
Zafirlukast CAS 107753-78-6View Details
107753-78-6 -
 Zafirlukast CAS 107753-78-6View Details
Zafirlukast CAS 107753-78-6View Details
107753-78-6 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
