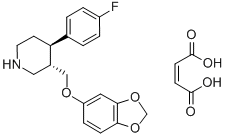
Paroxetine maleate
Synonym(s):(3S-trans)-3-[(1,3-Benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine maleate salt;BRL-29060;FG-7051
- CAS NO.:64006-44-6
- Empirical Formula: C23H24FNO7
- Molecular Weight: 445.44
- MDL number: MFCD01742665
- EINECS: 636-343-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 20:41:25
What is Paroxetine maleate?
The Uses of Paroxetine maleate
Paroxetine maleate is a selective serotonin uptake inhibitor.
What are the applications of Application
Paroxetine maleate is a selective serotonin uptake inhibitor
Definition
ChEBI: A maleate salt obtained by reaction of paroxetine with one equivalent of maleic acid. Highly potent and selective 5-HT uptake inhibitor that binds with high affinity to the serotonin transporter (Ki = 0.05 nM). Ki values are 1.1, 350 and 1100 nM for inhibi ion of [3H]-5-HT, [3H]-l-NA and [3H]-DA uptake respectively. Displays minimal affinity for alpha1-, alpha2- or beta-adrenoceptors, 5-HT2A, 5-HT1A, D2
General Description
Paroxetine, marketed under trade names such as Paxil? or Aropax, is an SSRI antidepressant used to treat many conditions in adults from major depression and obsessive-compulsive disorder to several anxiety disorders. Suitable uses for this certified solution standard include as a starting material for calibrators and controls in LC/MS or GC/MS paroxetine testing applications such as urine drug testing, prescription monitoring, clinical toxicology, or forensic analysis.
Biological Activity
Highly potent and selective 5-HT uptake inhibitor that binds with high affinity to the serotonin transporter (K i = 0.05 nM). K i values are 1.1, 350 and 1100 nM for inhibition of [ 3 H]-5-HT, [ 3 H]-l-NA and [ 3 H]-DA uptake respectively. Displays minimal affinity for a 1 -, a 2 - or b-adrenoceptors, 5-HT 2A , 5-HT 1A , D 2 or H 1 receptors at concentrations below 1000 nM, however displays weak affinity for muscarinic ACh receptors (K i = 42 nM). Antidepressant and anxiolytic in vivo .
Biochem/physiol Actions
Paroxetine is a strong cytochrome P450 2D6 isotype (CYP2D6) inhibitor, which reduces the effectiveness of tamoxifen. This phenylpiperidine derivative inhibits clozapine metabolism. Paroxetine is used to treat social phobia, obsessive-compulsive disorder and panic disorder. It is also used to treat the premenstrual dysphoric disorder, post-traumatic stress disorder and chronic headache.
storage
Store at RT
Properties of Paroxetine maleate
| Melting point: | 136-138°C |
| alpha | D -87° (c = 5 in ethanol) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | DMSO: ~12 mg/mL |
| form | solid |
| color | white |
Safety information for Paroxetine maleate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Paroxetine maleate
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Paroxetine maleate solution CAS 64006-44-6View Details
Paroxetine maleate solution CAS 64006-44-6View Details
64006-44-6 -
 Paroxetine maleate salt CAS 64006-44-6View Details
Paroxetine maleate salt CAS 64006-44-6View Details
64006-44-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1