Methyltrimethoxysilane
Synonym(s):Methyltrimethoxysilane;MTMS;Trimethoxymethylsilane
- CAS NO.:1185-55-3
- Empirical Formula: C4H12O3Si
- Molecular Weight: 136.22
- MDL number: MFCD00008342
- EINECS: 214-685-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
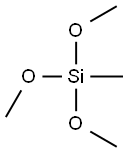
What is Methyltrimethoxysilane?
Description
Trimethoxy (methyl) silane is an organosilicon compound. It can be used as a crosslinker in the preparation of polysiloxane polymers. It can also be used as an acid scavenge used in the formation of substituted azulenes from allenylsilanes and tropyl?ium tetrafluoroborate. It can also be used as the precursor for synthesis of flexible silica aerogels.
Chemical properties
Colorless clear liquid
The Uses of Methyltrimethoxysilane
Methyltrimethoxysilane(MTMS) in combination with iron nitrate altered the pore structure dramatically. As the Crosslinking agent of RTV silicone rubber and glass fiber surface treatment agent and talk to agents outside of reinforced plastic laminated products in order to improve the mechanical strength, heat resistance, moisture resistance. Methyltrimethoxysilane is used as an acid scavenger, for example in the formation of substituted azulenes from allenylsilanes and tropyl-ium tetrafluoroborate.
The Uses of Methyltrimethoxysilane
Methyltrimethoxysilane is a reagent used in they synthesis of electronic materials and organometallic compounds. Used in the coating of carbon-fiber surfaces, as well as in the synthesis of nanocomposites.
What are the applications of Application
Trimethoxymethylsilane is an organosilicon compound used as a monomer in the production of silicone polymers and resins
What are the applications of Application
Methyltrimethoxysilane is highly miscible with standard organic solvents, such as alcohols, hydrocarbons and acetone. It is practically insoluble in neutral water and reacts only slowly to form silanols and higher condensation products. Addition of a hydrolytic catalyst (inorganic/organic acids, ammonia or amines) accelerates the hydrolysis of Methyltrimethoxysilane substantially.
As a Filler Modifier, Methyltrimethoxysilane is used mainly to render a wide range of surfaces and materials water repellent (e.g. mineral fillers, pigments, glass, cardboard). It may be used pure or in solution to treat fillers, using suitable mixing equipment. It may be necessary to first pre-treat the substrate with water and/or a catalyst. It is also used in the production of silicone resins and condensation-curing silicone rubber, used as an important component in sol-gel systems.
As one of the most common Alkoxy Crosslinkers, Methyltrimethoxysilane has high reactivity that precedes by nucleophilic substitution usually in the presence of acid or base catalysts.
Preparation
Methyltrimethoxysilane is usually prepared from methyltrichlorosilane and methanol:
CH3SiCl3 + 3 CH3OH → CH3Si(OCH3)3 + 3 HCl
General Description
Trimethoxymethylsilane (MTM) is an organosilicon compound widely used as a precursor for the preparation of silica-based materials, which finds the applications in various fields. Particularly in molecular assembly, linking nano building blocks, and selective synthesis oligosiloxane compounds. It can also be utilized as a crosslinker in the synthesis of polysiloxane polymers.
Flammability and Explosibility
Flammable
Purification Methods
Methyl trimethoxysilane [1185-55-3] M 136.2, b 102o/760mm, d 4 1.3687, n D 1.3711. Likely impurities are 1,3-dimethyltetramethoxy disiloxane (b 31o/1mm) and cyclic polysiloxanes, see methyl triethoxysilane. [Tamborski & Post J Org Chem 16 1400 1952, Seyferth & Rochow J Org Chem 20 250 1955, Beilstein 4 IV 4203.]
References
https://en.wikipedia.org/wiki/Methyltrimethoxysilane https://www.alfa.com/zh-cn/catalog/B23594/
Rao, A. V., et al. "Synthesis of flexible silica aerogels using methyltrimethoxysilane (MTMS) precursor." Journal of Colloid & Interface Science 300.1(2006):279-85.
Bhagat, Sharad D., et al. "Methyltrimethoxysilane based monolithic silica aerogels via ambient pressure drying." Microporous & Mesoporous Materials 100.1–3(2007):350-355.
Properties of Methyltrimethoxysilane
| Melting point: | <-70°C |
| Boiling point: | 102-104 °C(lit.) |
| Density | 0.955 g/mL at 25 °C(lit.) |
| vapor pressure | 2990 hPa (20 °C) |
| refractive index | n |
| Flash point: | 52 °F |
| storage temp. | Store below +30°C. |
| form | liquid |
| Specific Gravity | 0.955 |
| color | colorless |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| BRN | 1736151 |
| Stability: | Stable, but moisture sensitive. Highly flammable. Incompatible with water, strong acids, strong oxidizing agents. |
| CAS DataBase Reference | 1185-55-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Silane, trimethoxymethyl-(1185-55-3) |
| EPA Substance Registry System | Trimethoxymethylsilane (1185-55-3) |
Safety information for Methyltrimethoxysilane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H225:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P243:Take precautionary measures against static discharge. |
Computed Descriptors for Methyltrimethoxysilane
| InChIKey | BFXIKLCIZHOAAZ-UHFFFAOYSA-N |
New Products
BOC-L-4-HYDROXYPROLINE 2-nitro 3-hydroxy pyridine 2,6-Dichloropyridin-4-amine 2,3 Diamino pyridine 5-Iodo-2-(1-methylethyl)-3(2H)-pyridazinone 1-Azetidinecarboxylic acid, 3-[(3S)-1-(trans-3-carboxy-3-methylcyclobutyl)-3-piperidinyl]-, 1-(1,1-dimethylethyl) ester Tert-Butyl N-[3-(dimethylcarbamoyl)prop-2-en-1-yl]carbamate 1-(difluoromethyl)-N-methylcyclobutan-1-amine 2-(4-Methyl-1,2,5-oxadiazol-3-yl)-1H-benzimidazole 6-(4-iodophenyl)-1-oxa-6-azaspiro[3.3]he Trimethyl(phenylthio)silane Polycaprolactone(2000)-PEG(20000)-Polycaprolactone(2000) Diacrylate Diethylene Glycol Monoethyl Ether, PolyoxyethyleneOleylCetylEtherSulfosuccinate Ascorbyl Tetraisopalmitate or Tetrahexyldecyl Ascorbate Castor Oil, Ethoxylated, Cremophor EL or PEG-35 Castor Oil Tween 20 or Polysorbate 20 Acetone-d6 (R)-2-Mercaptobutanoic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine 3-(naphthalen-1-ylsulfonyl)-1H-indazol-5-amine methyl 5-amino-3-(1,1-dioxidotetrahydro-2H-1,2-thiazin-2-yl)-2-fluorobenzoate 7-methoxy-8-(2-morpholinoethoxy)-4-((3,4,5-trimethoxyphenyl)amino)benzo[g]quinoline-3-carbonitrile Dimethylaluminum isopropoxideRelated products of tetrahydrofuran
![1,2-Ethanediamine,N-[(trimethoxysilyl)methyl]-](https://img.chemicalbook.in/CAS/GIF/51980-40-6.gif)
You may like
-
 1185-55-3 METHYL TRIMETHOXY SILANE 98%View Details
1185-55-3 METHYL TRIMETHOXY SILANE 98%View Details
1185-55-3 -
 Methyltrimethoxysilane CAS 1185-55-3View Details
Methyltrimethoxysilane CAS 1185-55-3View Details
1185-55-3 -
 Methyltrimethoxysilane 98% CAS 1185-55-3View Details
Methyltrimethoxysilane 98% CAS 1185-55-3View Details
1185-55-3 -
 Trimethoxy(methyl)silane CAS 1185-55-3View Details
Trimethoxy(methyl)silane CAS 1185-55-3View Details
1185-55-3 -
 Trimethoxymethylsilane CAS 1185-55-3View Details
Trimethoxymethylsilane CAS 1185-55-3View Details
1185-55-3 -
 Trimethoxymethylsilane CAS 1185-55-3View Details
Trimethoxymethylsilane CAS 1185-55-3View Details
1185-55-3 -
 Trimethoxymethylsilane CAS 1185-55-3View Details
Trimethoxymethylsilane CAS 1185-55-3View Details
1185-55-3 -
 Trimethoxymethylsilane CAS 1185-55-3View Details
Trimethoxymethylsilane CAS 1185-55-3View Details
1185-55-3
