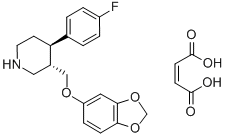
64006-44-6
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 445.4 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 6 |
| Exact Mass | 445.15368027 g/mol |
| Monoisotopic Mass | 445.15368027 g/mol |
| Topological Polar Surface Area | 114 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 521 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Paroxetine maleate is a maleate salt obtained by reaction of paroxetine with one equivalent of maleic acid. Highly potent and selective 5-HT uptake inhibitor that binds with high affinity to the serotonin transporter (Ki = 0.05 nM). Ki values are 1.1, 350 and 1100 nM for inhibition of [3H]-5-HT, [3H]-l-NA and [3H]-DA uptake respectively. Displays minimal affinity for alpha1-, alpha2- or beta-adrenoceptors, 5-HT2A, 5-HT1A, D2 or H1 receptors at concentrations below 1000 nM, however displays weak affinity for muscarinic ACh receptors (Ki = 42 nM). Antidepressant and anxiolytic in vivo. It has a role as an antidepressant, an anxiolytic drug, a serotonin uptake inhibitor, a hepatotoxic agent and a P450 inhibitor. It contains a paroxetinium(1+).