Palonosetron
- CAS NO.:135729-61-2
- Empirical Formula: C19H24N2O
- Molecular Weight: 296.41
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
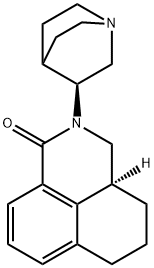
What is Palonosetron?
Description
Palonosetron is a new generation 5-HT3 antagonist and It has hasgreater receptor-binding properties, which results in a much longer half-life than the previously described drugs. This selective and conformationally restricted 5-HT3 receptor antagonist was approved for the treatment of chemotherapy-induced nausea and vomiting. The drug was originally developed by Syntex Corp (now Roche Bioscience) and is currently being developed by Helsinn and MGI Pharm.
The Uses of Palonosetron
Palonosetron is a serotonin 5-HT3 receptor antagonist used in the prevention and treatment of chemotherapy-induced nausea and vomiting (CINV). Antiemetic.
Definition
ChEBI: An organic heterotricyclic compound that is an antiemetic used (as its hydrochloride salt) in combination with netupitant (under the trade name Akynzeo) to treat nausea and vomiting in patients undergoing cancer chemotherapy.
Clinical Use
Anti-emetic:
For use with cancer chemotherapy
Synthesis
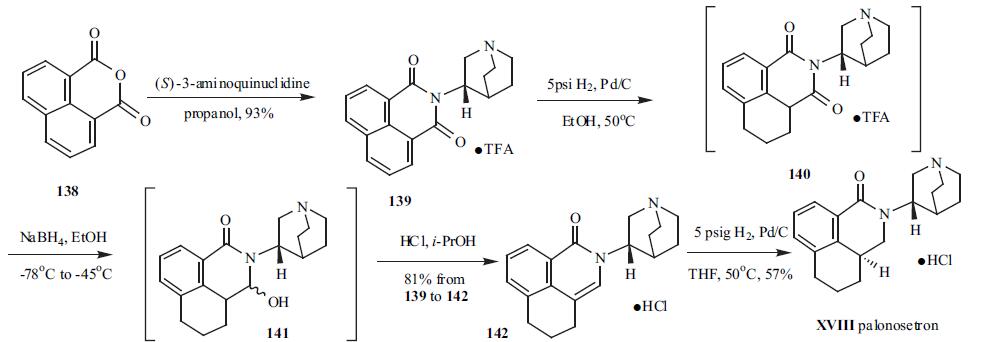
(S)-3-Aminoquinuclidine was condensed with inexpensive 1,8-naphthalic anhydride (138) to furnish imide 139 in 93% yield and isolated as its TFA salt. Imide 139 was hydrogenated at 5 psi to give intermediate 140 with one of the reduced aromatic ring. The less hindered C-3 carbonyl group in 140 was selectively reduced to a hydroxy group by using sodium borohydride in ethanol under nitrogen at low temperature to give intermediate 141. Intermediate 141 was not isolated because of the formation of a tight boron complex. Subsequently, acid was added to 141 in i-PrOH to decompose the boron complex and dehydrate intermediate 141 to 142, which was conveniently isolated as its HCl salt in 75% yield from 139. Palonosetron (XVIII) was obtained in 57% yield by palladium-catalyzed hydrogenation of 142.
Enzyme inhibitor
This serotonin 5-HT3 receptor antagonist and longlasting anti-emetic (FW = 296.41 g/mol; CAS 135729-61-2), known by code name RS 25259, RS 25259-197, trade name Aloxi?, and systematic name (3aS)-2-[(3S)-1- azabicyclo[2.2.2]oct-3-yl]-2,3,3a,4,5,6-hexahydro-1H-benz[de]isoquinolin- 1-one, is used to treat chemotherapy-induced nausea/vomiting. Polonosetron affects both peripheral and central nervous systems, reducing vagus nerve activity, thereby deactivating the 5-HT3 receptor-dense vomiting center located in the medulla oblongata. It is without effect on dopamine receptors or muscarinic receptors. Primary Mode of Inhibitor Action: Intravenously administered palonosetron has a linear pharmacokinetic profile, with a long terminal elimination half-life of ~40 hours and moderate (62%) plasma protein binding. Its high affinity and long half-life most likely explains its persistent antiemetic effect. Similar 5- HT3 antagonists include tropisetron, ondansetron, dolasetron, and granisetron. The effectiveness of each depends on particular variants of 5- HT3 receptors expressed by the patient, including changes in promoters for the receptor genes
Metabolism
Palonosetron is eliminated by a dual route, about 40%
eliminated through the kidney and approximately
50% metabolised by CYP2D6, and to a lesser extent,
CYP3A4 and CYP1A2 isoenzymes in the liver to form
two primary metabolites, which have less than 1% of
the 5HT3
receptor antagonist activity of palonosetron.
After a single intravenous dose of [14C]-palonosetron,
approximately 80% of the dose was recovered within
144 hours in the urine with unchanged palonosetron
representing approximately 40% of the administered dose.
Properties of Palonosetron
| Melting point: | 87-88° |
| Boiling point: | 470.4±45.0 °C(Predicted) |
| alpha | D -136° (c = 0.25 in chloroform) |
| Density | 1.24±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO |
| form | Powder |
| pka | 9.77±0.33(Predicted) |
Safety information for Palonosetron
Computed Descriptors for Palonosetron
Palonosetron manufacturer
Om Pharmaceutical Industries
Aspen Biopharma Labs Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 135729-61-2 Palonosetron 99%View Details
135729-61-2 Palonosetron 99%View Details
135729-61-2 -
 135729-61-2 98%View Details
135729-61-2 98%View Details
135729-61-2 -
 Palonosetron 95% CAS 135729-61-2View Details
Palonosetron 95% CAS 135729-61-2View Details
135729-61-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1