Pramipexole
- CAS NO.:104632-26-0
- Empirical Formula: C10H17N3S
- Molecular Weight: 211.33
- MDL number: MFCD00869076
- EINECS: 600-593-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
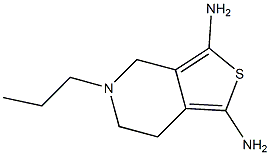
What is Pramipexole?
Absorption
The bioavailability of pramipexole is higher than 90%, indicating a high level of absorption .
Toxicity
LD50
Rat Oral LD 50 >800 mg/kg .
Carcinogenicity, mutagenicity, impact on fertility
Pramipexole was not found to be carcinogenic in 2-year studies on mice and rats at 0.3, 2.2, and 11 times the maximum recommended human dose (MRHD). No increased incidence of tumors was observed . No mutagenicity was detected in various assays, including the Ames test. Finally, pramipexole given to rat models at a dose of 2.5 mg/kg/day (5 times the maximum recommended human dose), increased estrus cycles and inhibited implantation of a fertilized ovum. Decreased levels of prolactin, a hormone necessary for implantation and maintenance of early pregnancy, were measured . The significance of these findings in humans is unknown.
Pregnancy
This drug is considered a pregnancy category C drug, showing teratogenic effects in animals. Currently, there no studies of pramipexole in human pregnancy. Animal reproduction studies are not always predictive of human response. This drug should only be used in pregnancy if the potential benefit outweighs the possible fetal risks .
Nursing
Whether pramipexole is excreted in human milk is unknown. A decision should be made regarding the administration pramipexole during nursing, or whether to discontinue it during nursing, as many drugs are excreted in human milk. The potential exists for risk to the infant if pramipexole is, in fact, excreted in the milk .
Chemical properties
Off-White Solid
Originator
Mirapex, Pharmacia and Upjohn, USA
The Uses of Pramipexole
(S)-Pramipexole enantiomer. A dopamine-D2-receptor agonist. Antiparkinsonian
The Uses of Pramipexole
partial/full D2S, D2L, D3, D4 receptor agonist
The Uses of Pramipexole
(S)-Pramipexole enantiomer. s disease (PD) and restless legs syndrome (RLS).[1] It is also sometimes used off-label as a treatment for cluster headache and to counteract the problems with sexual dysfunction experienced by some u sers of the selective serotonin reuptake inhibitor (SSRI) antidepressants.
Indications
This drug is indicated for the symptomatic treatment of Parkinson’s disease . This drug can be administered as monotherapy or in conjunction with levodopa. It is also indicated for symptomatic treatment of moderate to severe primary Restless Legs Syndrome (RLS) .
Background
Pramipexole is a drug used to treat the symptoms of Parkinson's Disease (PD). It is a non-ergot dopamine agonist drug that is efficacious in treating various Parkinson's symptoms such as tremor, rigidity, and bradykinesia (slow movement) . It was first approved by the FDA in 1997 . Parkinson's Disease is one of the most common neurodegenerative disorders and causes a high level of disability in patients , leading to increased difficulty in performing activities of daily living due to symptoms that progress over time . The prevalence of Parkinson's Disease worldwide has increased from approximately 2.5 million in 1990 to about 6.1 million in 2016 . This increase may be attributed to an aging population along with other contributing factors .
In addition to the above FDA approval for Parkinson's Disease, pramipexole was also approved by the FDA in 2006 for the treatment of Restless Legs Syndrome (RLS) . RLS is a sleep-related disorder characterized by unpleasant sensations in the lower extremities, often accompanied by an uncontrollable urge to move the legs .
Definition
ChEBI: A member of the class of benzothiazoles that is 4,5,6,7-tetrahydro-1,3-benzothiazole in which the hydrogens at the 2 and 6-pro-S-positions are substituted by amino and propylamino groups, respectively.
Manufacturing Process
75.5 g (0.5 mol) of 4-aminocyclohexanol hydrochloride and 74.0 g (0.5 mol) of phthalic acid anhydride are mixed with 65 g (0.5 mol) of ethyldiisopropyl amine and 1000 ml of toluene and boiled for 36 hours with a water separator. Then water is added, the toluene phase is separated off and the aqueous phase is extracted several times with chloroform. The organic phases are combined, dried and concentrated. The concentrated residue is recrystallised from isopropanol and 4-(phthalimido)-cyclohexanol was obtained. Yield: 95 g (77.8%). Melting point 175°-176°C.
95 g (0.388 mol) of 4-(phthalimido)-cyclohexanol are dissolved in 600 ml of chloroform and, after the addition of 450 ml of water and 120 ml of sulfuric acid, 90 g (0.3 mol) of potassium dichromate are added in batches. The internal temperature of the mixture is maintained at between 25° and 30°C by slight cooling. The mixture is stirred for a further 3 hours, then the chloroform phase is separated off and the mixture extracted twice more with chloroform. After drying and concentration of the extracts 82 g (86.9%) of 4(phthalimido)-cyclohexanone was obtained.
48.6 g (0.2 mol) of 4-(phthalimido)cyclohexanone are dissolved in glacial acetic acid, mixed with 36% of hydrobromic acid in glacial acetic acid and then 32 g (0.2 mol) of bromine in glacial acetic acid is added dropwise with cooling. The mixture is then concentrated by evaporation in vacuo and the residue is triturated several times with diethylether. The ether extracts are discarded and the residue is dissolved in of ethanol. After thiourea have been added the mixture is refluxed for 5 hours. It is then concentrated by evaporation, made alkaline with sodium hydroxide solution and extracted with chloroform. After drying and concentration of the extracts, the residue is purified by column chromatography on silica gel (eluant: chloroform/methanol = 1/1). The 2-amino-6-phthalimido-4,5,6,7-tetrahydro-benzthiazol was obtained. Melting point 244-246°C, dec. Yield: 30 g (50%).
9.5 g (31.7 mmol) of 2-amino-6-phthalimido-4,5,6,7-tetrahydro-benzthiazole are suspended in 100 ml of ethanol and, after the addition of 1.8 g (36 mmol) of hydrazine hydrate, refluxed for 2 hours. The mixture is then concentrated and purified by column chromatography on silica gel using methanol as eluant. The 2,6-diamino- 4,5,6,7-tetrahydro-benzthiazole was obtained.
To a solution of 2,6-diamino- 4,5,6,7-tetrahydro-benzthiazole in dimethylformamide are added n-propanal and the mixture is heated to 50°C for 1 hour. After cooling, the reaction solution is mixed with sodium borohydride and heated to 50°C for 30 min. The solvent is largely eliminated in vacuo. Whilst cooling with ice, the residue is mixed with water and 2 N hydrochloric acid until a pH of 1 is obtained. The aqueous solution is exwith ethylacetate and the organic phase discarded. The aqueous phase is mixed with potassium carbonate until an alkaline reaction is obtained and then extracted with ethyl acetate. The organic phase is dried and concentrated. The 2-amino-6-n-propylamino-4,5,6,7-tetrahydro-benzthiazole dihydrochloride crystallizes out when ethereal hydrochloric acid is added. Yield: 42%. Melting point: 286°-288°C.
Therapeutic Function
Antiparkinsonian, Antipsychotic
Biological Activity
pramipexole is a dopamine agonist of the non-ergoline class indicated for treating parkinson's disease (pd) and restless legs syndrome (rls).pramipexole also possesses low/insignificant affinity (500-10,000 nm) for the 5-ht1a, 5-ht1b, 5-ht1d, and α2-adren
Pharmacokinetics
Parkinson's Disease
Through the stimulation of dopamine receptors, pramipexole is thought to relieve the symptoms of Parkinson's Disease . The motor symptoms of Parkinson's disease occur partly due to a reduction of dopamine in the substantia nigra of the brain . Dopamine is an essential neurotransmitter that has major effects on motor movements in humans.
Restless Legs Syndrome
Pramipexole likely restores balance to the dopaminergic system, controlling the symptoms of this condition. Restless legs syndrome is thought to occur, in part, through dysfunction of the dopaminergic system, resulting in unpleasant lower extremity symptoms , .
Other effects
In addition to the abovementioned effects, animal studies demonstrate that pramipexole blocks dopamine synthesis, release, and turnover. Additionally, this drug is neuroprotective to dopamine neuron degeneration after ischemia or methamphetamine neurotoxicity .
Metabolism
This drug undergoes little metabolism in humans .
Properties of Pramipexole
| Melting point: | 288-290°C |
| Boiling point: | 378.0±42.0 °C(Predicted) |
| Density | 1.17±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 9.47±0.20(Predicted) |
| color | White to Off-White |
| CAS DataBase Reference | 104632-26-0(CAS DataBase Reference) |
Safety information for Pramipexole
Computed Descriptors for Pramipexole
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 N(6)-Propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine dihydrochloride 95% CAS 104632-26-0View Details
N(6)-Propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine dihydrochloride 95% CAS 104632-26-0View Details
104632-26-0 -
 Pramipexole 98% CAS 104632-26-0View Details
Pramipexole 98% CAS 104632-26-0View Details
104632-26-0 -
 Pramipexole API Powder, Analytical Grade, 25kgView Details
Pramipexole API Powder, Analytical Grade, 25kgView Details
104632-26-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
