p-Toluidine
Synonym(s):p-Toluidine;4-Aminotoluene;4-Aminotoluene, 4-Methylaniline;4-Methylaniline
- CAS NO.:106-49-0
- Empirical Formula: C7H9N
- Molecular Weight: 107.15
- MDL number: MFCD00007906
- EINECS: 203-403-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
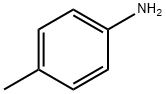
What is p-Toluidine?
Chemical properties
White lustrous plates or leaflets. Soluble in alcohol and ether; very slightly soluble in water. Combustible.
Chemical properties
p-Toluidine is a colorless solid.
The Uses of p-Toluidine
p-Toluidine is used as an intermediate in themanufacture of various dyes. It is also usedas a reagent for lignin and nitrites.
The Uses of p-Toluidine
Manufacture of various dyes and other organic chemicals. o-Isomer also in printing textiles blue black; making colors fast to acids. p-Isomer also as a reagent for lignin, nitrite, phloroglucinol.
The Uses of p-Toluidine
p-Toluidine is an intermediate in the production of dyes, organic chemicals and aromatic azo compounds. It serves as a component of accelerators for cyanoacrylate glues. Furthermore, it acts as a bidentate Schiff base ligand through condensation with salicylaldehyde. It reacts with catecholamine to form a dye which is useful for spectrophotometric determination of catecholamine drugs.
Definition
ChEBI: An aminotoluene in which the amino substituent is para to the methyl group.
Synthesis Reference(s)
Tetrahedron Letters, 32, p. 2759, 1991 DOI: 10.1016/0040-4039(91)85078-J
Journal of the American Chemical Society, 89, p. 5311, 1967 DOI: 10.1021/ja00996a055
General Description
Colorless solid. Melting point 44°C (111°F). Specific gravity 1.046. Vapor heavier than air. Produces toxic oxides of nitrogen during combustion. May be absorbed through the skin. Used in dyes, and in organic chemical manufacturing.
Reactivity Profile
p-Toluidine neutralizes acids to form salts plus water in exothermic reactions. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated in combination with strong reducing agents, such as hydrides. Can react vigorously with oxidizing reagents. Emits very toxic fumes of oxides of nitrogen when heated to decomposition. Hypergolic reaction with red fuming nitric acid [Kit and Evered, 1960, p. 239, 242].
Health Hazard
Absorption of toxic quantities by any route causes cyanosis (blue discoloration of lips, nails, skin); nausea, vomiting, and coma may follow. Repeated inhalation of low concentrations may cause pallor, low-grade secondary anemia, fatigability, and loss of appetite. Contact with eyes causes irritation.
Health Hazard
p-Toluidine is a mild to moderate irritanton the skin. The irritant effect on rabbits’eyes was strong. The toxic properties ofp-toluidine are similar to its ortho- and meta isomers and aniline. The clinical signs of tox icity are methemoglobinemia, anemia, andcyanosis. The major metabolite in urine afteroral application in male rats was 2-amino-5-methylphenol, which was excreted alongwith 3.5% unchanged p-toluidine (ACGIH1986). Exposure to 40-ppm concentrationfor 1 hour resulted in severe poisoning inhumans.
Fire Hazard
Special Hazards of Combustion Products: Toxic and flammable vapors may form in fire.
Safety Profile
Confirmed carcinogen. Poison by ingestion and intraperitoneal routes. Mutation data reported. A severe skin and eye irritant. Flammable when exposed to heat, flame, or oxidizers. Can react vigorously on contact with oxidzing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits highly toxic fumes of NOx. See also o- TOLUIDINE and ANILINE.
Potential Exposure
para-Toluidine is used in dyes, and in organic chemical manufacturing
Carcinogenicity
In an 18-month p-toluidine hydrochloride diet carcinogenicity study, maleCDrats (1000 and 2000 ppmfor 18 months) did not develop statistically significant increases of tumors; however,CD-1male and female mice (1000 ppmfor 6 months and then 500 ppmfor additional 12 months; or 2000 ppm for 6 months and then 1000 ppm for additional 12 months) showed significant increases in liver carcinomas: in males at both dose levels and in females at the high dose level.
Shipping
UN3451 Toluidines, solid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
In general, methods similar to those for purifying aniline can be used. It can be separated from the o-and m-isomers by fractional crystallisation from its melt. p-Toluidine has been crystallised from hot water (charcoal), EtOH, *benzene, pet ether or EtOH/water (1:4), and dried in a vacuum desiccator. It can also be sublimed at 30o under vacuum. For further purification, use has been made of the oxalate, the sulfate and acetylation. The oxalate, formed as described for o-toluidine, is filtered, washed and recrystallised three times from hot distilled water. The base is regenerated with aqueous Na2CO3 and recrystallised three times from distilled water. [Berliner & May J Am Chem Soc 49 1007 1927.] Alternatively, p-toluidine is converted to its acetyl derivative which, after repeated crystallisation from EtOH, is hydrolysed by refluxing (50g) in a mixture of 500mL of water and 115mL of conc H2SO4 until a clear solution is obtained. The amine sulfate is isolated, suspended in water, and NaOH is added. The free base is distilled twice from zinc dust under vacuum. The p-toluidine is then recrystallised from pet ether and dried in a vacuum desiccator or in a vacuum for 6hours at 40o. The benzoyl derivative has m 158o (from EtOH). [Berliner & Berliner J Am Chem Soc 76 6179 1954, Moore et al. J Am Chem Soc 108 2257 1986, Beilstein 12 H 880, 12 I 140, 12 II 482, 12 III 2017, 12 IV 1866.]
Incompatibilities
para-Toluidine is incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. p-Toluidine neutralizes acids to form salts plus water in exothermic reactions. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated in combination with strong reducing agents, such as hydrides. Hypergolic reaction with red fuming nitric acid
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Controlled incineration (oxides of nitrogen are removed from the effluent gas by scrubbers and/or thermal devices).
Properties of p-Toluidine
| Melting point: | 41-46 °C(lit.) |
| Boiling point: | 200 °C(lit.) |
| Density | 0.973 g/mL at 25 °C(lit.) |
| vapor density | 3.9 (vs air) |
| vapor pressure | 0.26 mm Hg ( 25 °C) |
| refractive index | 1.5636 |
| Flash point: | 192 °F |
| storage temp. | Store below +30°C. |
| solubility | Soluble in ethanol, pyridine, diethyl ether, acetone, carbon tetrachloride, methanol, carbon disulfide, oils and dilute acids. |
| form | powder |
| pka | 5.08(at 25℃) |
| Specific Gravity | 0.962 |
| color | tan to brown |
| explosive limit | 6.6% |
| Water Solubility | 1.1 g/100 mL |
| Sensitive | Air & Light Sensitive |
| Merck | 14,9536 |
| BRN | 471281 |
| Exposure limits | TLV-TWA skin 2 ppm (~9 mg/m3) (ACGIH);
Suspected Human Carcinogen (ACGIH). |
| Dielectric constant | 4.9800000000000004 |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong acids. |
| CAS DataBase Reference | 106-49-0(CAS DataBase Reference) |
| NIST Chemistry Reference | p-Toluidine(106-49-0) |
| EPA Substance Registry System | p-Toluidine (106-49-0) |
Safety information for p-Toluidine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H351:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for p-Toluidine
p-Toluidine manufacturer
JSK Chemicals
CEFA CILINAS BIOTICS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 p-Toluidine 98%View Details
p-Toluidine 98%View Details -
 p-Toluidine 98%View Details
p-Toluidine 98%View Details -
 p-Toluidine CAS 106-49-0View Details
p-Toluidine CAS 106-49-0View Details
106-49-0 -
 p-Toluidine, GR 99%+ CAS 106-49-0View Details
p-Toluidine, GR 99%+ CAS 106-49-0View Details
106-49-0 -
 p-Toluidine CAS 106-49-0View Details
p-Toluidine CAS 106-49-0View Details
106-49-0 -
 p-TOLUIDINE For Synthesis CAS 106-49-0View Details
p-TOLUIDINE For Synthesis CAS 106-49-0View Details
106-49-0 -
 p-TOLUIDINE AR CAS 106-49-0View Details
p-TOLUIDINE AR CAS 106-49-0View Details
106-49-0 -
 Para Toluidine (PT), 106-49-0View Details
Para Toluidine (PT), 106-49-0View Details
106-49-0
