129-20-4
Product Name:
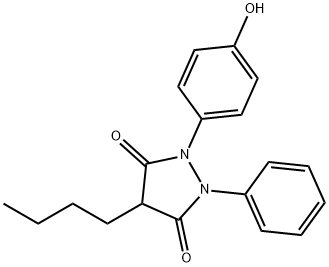
OXYPHENYL BUTAZONE
Formula:
C19H20N2O3
Synonyms:
4-Butyl-1-(4-hydroxyphenyl)-2-phenyl-3,5-pyrazolidinedione;p-Hydroxyphenylbutazone;p-Oxyphenylbutazone;
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | WHITE TO YELLOWISH WHITE, CRYSTALLINE POWDER |
|---|---|
| Odor | ODORLESS |
| Melting Point | 96 °C |
| Solubility | 60 mg/L (at 30 °C) |
| LogP | 2.72 |
| LogS | -3.73 |
| Collision Cross Section | 177.2 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
COMPUTED DESCRIPTORS
| Molecular Weight | 324.4 g/mol |
|---|---|
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 324.14739250 g/mol |
| Monoisotopic Mass | 324.14739250 g/mol |
| Topological Polar Surface Area | 60.8 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 454 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Oxyphenbutazone is a metabolite of phenylbutazone obtained by hydroxylation at position 4 of one of the phenyl rings. Commonly used (as its hydrate) to treat pain, swelling and stiffness associated with arthritis and gout, it was withdrawn from the market 1984 following association with blood dyscrasis and Stevens-Johnson syndrome. It has a role as a non-steroidal anti-inflammatory drug, a non-narcotic analgesic, an antipyretic, a gout suppressant, a drug metabolite, a xenobiotic metabolite, an antineoplastic agent and an antimicrobial agent. It is a member of pyrazolidines and a member of phenols. It is functionally related to a phenylbutazone.