Oxaprozin
Synonym(s):4,5-Diphenyl-2-oxazolepropanoic acid;Daypro;Oxaprozin
- CAS NO.:21256-18-8
- Empirical Formula: C18H15NO3
- Molecular Weight: 293.32
- MDL number: MFCD00215977
- EINECS: 244-296-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
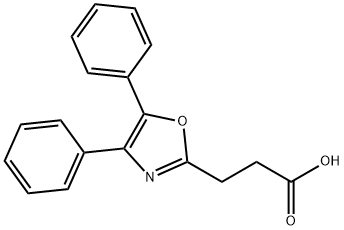
What is Oxaprozin?
Absorption
Oxaprozin is 95% absorbed after oral administration. Food may reduce the rate of absorption of oxaprozin, but the extent of absorption is unchanged. Antacids do not significantly affect the extent and rate of oxaprozin absorption.
Toxicity
Oral, mouse: LD50 = 1210 mg/kg; Oral, rabbit: LD50 = 172 mg/kg; Oral, rat: LD50 = 4470 mg/kg
Description
Oxaprozin is a non-steroidal antiinflammatory agent indicated for use in various forms of rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis. A long T1/2 allows once/twice daily dosing. Structurally it is somewhat unique, being a 3- substituted, rather than a 2-substituted propionic acid.
Chemical properties
White Solid
Originator
Wyeth (United Kingdom)
The Uses of Oxaprozin
Oxaprozin is an anti-inflammatory drug. Oxaprozin was comparable to Aspirin (A687780).
The Uses of Oxaprozin
antiparasitic
Indications
Used to relieve the inflammation, swelling, stiffness, and joint pain associated with rheumatoid arthritis and osteoarthritis.
Background
Oxaprozin is a non-narcotic, non-steroidal anti-inflammatory drug (NSAID), used to relieve the inflammation, swelling, stiffness, and joint pain associated with osteoarthritis and rheumatoid arthritis.
What are the applications of Application
Oxaprozin is a non-narcotic anti-inflammatory agent shown to inhibit prostaglandin synthesis
Definition
ChEBI: A monocarboxylic acid that is a propionic acid derivative having a 4,5-diphenyl-1,3-oxazol-2-yl substituent at position 3. It is non-steroidal anti-inflammatory drug commonly used to relieve the pain and inflammatory responses associated with osteoarthrit s and rheumatoid arthritis.
Indications
Oxaprozin (Daypro) is approved for the treatment of osteoarthritis and rheumatoid arthritis. Its long halflife allows for once daily dosing.The most frequently reported adverse effects of this drug are nausea, vomiting, and dyspepsia.
Manufacturing Process
A clean dry reactor of 20 gallon (91 liters) capacity was charged with pyridine (9.25 kg), benzoin (16.5 kg) and succinic anhydride (11.7 kg.). The reactor was purged with nitrogen and a nitrogen atmosphere was maintain throughout the process. The mixture was heated without agitation until it became liquid at 85°C. Agitation was commenced and the mixture was heated at 90°-95°C for 1.5 hours. A solution of ammonium acetate (12.0 kg) in glacial acetic acid (35.0 kg) was charged to the header of the reactor and added to the reaction mixture over 15 minutes, maintaining the temperature between 90° and 95°C. The container for the solution and the header were washed with glacial acetic acid (4.0 kg) and the washing liquid was added to the reaction mixture. The reaction mixture was held at 90°-95°C for 2 hours. The reaction mixture was cooled to 50°C and transferred via a line filter to a reactor of 50 gallon (227 liters) capacity. The first reactor, lines and filter were washed with glacial acetic acid (4.0 kg.) which was combined with the reaction mixture. The reaction mixture was heated with agitation to 90°-95°C over 30 minutes and water (21.0 kg.) was added maintaining the temperature at 90°-95°C. The reaction mixture was then cooled to 20°-25°C over 55 minutes by means of water in the jacket of the reactor and then cooled to 10°-15°C by means of brine in the jacket and left overnight. The product was filtered on a ceramic filter and sucked well dry. The product on the filter was washed with a prefiltered mixture of glacial acetic acid (25.5 kg.) and water (12.5 kg) and
sucked well dry. Pre-filtered water (50.0 kg) and the filter cake were added to a reactor of 50 gallon (227 liters) capacity. The mixture was stirred at room temperature for 30 minutes and filtered on a ceramic filter and the product was sucked well dry. The product on the filter was washed twice with prefiltered water (10 kg each time) and sucked well dry. The product was then dried in a Mitchell oven at 80°C for 16-18 hours. The yield of crude β-(4,5diphenyloxazol-2-yl)propionic acid was 15.9 kg (69.8%). This material only just failed specification for acceptable purity because although TLC analysis showed only very faint trace impurities.
Recrystallisation of crude β-(4,5-Diphenyloxazol-2-yl)propionic acid
Methanol (62.0 kg) was added to a reactor of 50 gallon capacity (227 liters). 15.9 kg of the crude oxazole above prepared was added with agitation. The mixture was heated to reflux. All the solid dissolved. The mixture was then cooled to 50°C and transferred to a reactor of 20 gallon (91 liters) capacity. The larger reactor and transfer lines were washed through with methanol at about 40°C twice (3 kg each time). The mixture was cooled over 1 hour 50 minutes with agitation, gradually at first, to 15°-20°C by means of cooling water on the jacket of the reactor. The product was then filtered on a ceramic filter and sucked well dry. The product on the filter was washed twice with methanol (5 kg each time) and sucked well dry. The wash liquors were combined with the filtration liquors and retained. The product from the filter was dried in an air oven at 55°-60°C for 18 hours. The yield of β-(4,5diphenyloxazol-2-yl)propionic acid was 12.1 kg. TLC investigation showed the product to be pure. Melting point 160.5°-161.5°C.
Another crop of product was obtained from the methanol liquors as follows. The liquors were added to a reactor of 20 gallon (91 liters) capacity and the solvent was distilled off for 9 hours until solid appeared. The mixture was then cooled to 15° to 20°C over 1 3/4 hours using cooling water in the jacket of the reactor. The mixture was cooled to 10°C using brine in the jacket and stirred at this temperature for 30 minutes. The product was then filtered on a ceramic filter and sucked well dry. The product on the filter was washed twice with methanol (5 kg each time) and sucked well dry. The product was dried in an air oven at 55°-60°C for 18 hours. The yield was 2.14 kg. This product may also have been acceptably pure but its purity was not investigated. It was therefore retained as crude product to be resubjected to recrystallisation with methanol. The yield for the recrystallisation was thus 12.1 kg from a consumption of 13.76 kg of crude product, that is 88%. The overall yield of pure product is 69.8% times 88%, that is, 61.4%.
brand name
Daypro (Searle);DURAPROX.
Therapeutic Function
Antiinflammatory
General Description
Oxaprozin, 4,5-diphenyl-2-oxazolepropionic acid (Daypro),differs from the other members of this group in being anarylpropionic acid derivative. It shares the same propertiesand side effects of other members in this group. It is indicatedfor the short- and long-term management of OA andRA, administered as a once-daily dose of 600- to 1,200-mgdose because of its long duration of action.
Pharmacokinetics
Oxaprozin is a nonsteroidal anti-inflammatory drug (NSAID) with analgesic and antipyretic properties. Oxaprozin is used to treat rheumatoid arthritis, osteoarthritis, dysmenorrhea, and to alleviate moderate pain.
Pharmacokinetics
Oxaprozin is well absorbed (100%) following oral administration, but maximum plasma concentrations are not reached until 3 to 5 hours following ingestion. Oxaprozin is an anti-inflammatory agent possessing a rapid onset of action and a prolonged duration of action. In both the carrageenan raw paw edema assay and analgetic tests, it was equipotent with aspirin. Oxaprozin has been associated with the appearance of rash and/or mild photosensitivity. Some patients experience an increased incidence of rash on sun-exposed skin during clinical testing.
Clinical Use
Oxaprozine is a nonsteroidal anti-inflammatory drug used for the treatment of mild to moderate pain including rheumatoid arthritis and osteoarthritis. Oxaprozine shows slow elimination kinetics with an elimination half-life of about 24 h. It is given orally (600–1200 mg/d, maximum dose 1800 mg/d).
Safety Profile
Poison by ingestion, intravenous, and intraperitoneal routes. Moderately toxic by subcutaneous route. An experimental teratogen. Experimental reproductive effects. An anti-inflammatory agent. When heated to decomposition it emits toxic fumes of NOx.
Metabolism
Hepatic. Ester and ether glucuronide are the major conjugated metabolites of oxaprozin, and do not have significant pharmacologic activity.
References
1) Merck 14:6924 2) Ottonello et al. (2009), Delayed apoptosis of human monocytes exposed to immune complexes is reversed by oxaprozin: role of the Akt/IkappaB kinase/nuclear factor kappaB pathway; Br. .J. Pharmacol., 157 294 3) Dallegri et al. (2005), A review of the emerging profile of the anti-inflammatory drug oxaprozin; Expert Opin .Pharmacother., 6 777
Properties of Oxaprozin
| Melting point: | 154 °C |
| Boiling point: | 435.17°C (rough estimate) |
| Density | 1.2278 (rough estimate) |
| refractive index | 1.5100 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO |
| form | solid |
| pka | 4.3(at 25℃) |
| color | Crystals from methanol |
| Water Solubility | 4mg/L(37 ºC) |
| Merck | 14,6924 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 21256-18-8(CAS DataBase Reference) |
| EPA Substance Registry System | 2-Oxazolepropanoic acid, 4,5-diphenyl- (21256-18-8) |
Safety information for Oxaprozin
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Oxaprozin
| InChIKey | OFPXSFXSNFPTHF-UHFFFAOYSA-N |
Oxaprozin manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Oxaprozin CAS 21256-18-8View Details
Oxaprozin CAS 21256-18-8View Details
21256-18-8 -
 Oxaprozin 98% CAS 21256-18-8View Details
Oxaprozin 98% CAS 21256-18-8View Details
21256-18-8 -
 Oxaprozin CAS 21256-18-8View Details
Oxaprozin CAS 21256-18-8View Details
21256-18-8 -
 Oxaprozin CAS 21256-18-8View Details
Oxaprozin CAS 21256-18-8View Details
21256-18-8 -
 Oxaprozin CAS 21256-18-8View Details
Oxaprozin CAS 21256-18-8View Details
21256-18-8 -
 21256-18-8 Oxaprozin 98%View Details
21256-18-8 Oxaprozin 98%View Details
21256-18-8 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1