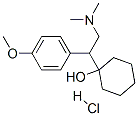
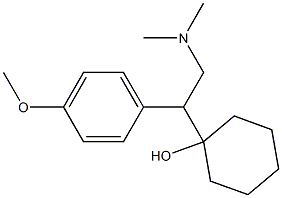
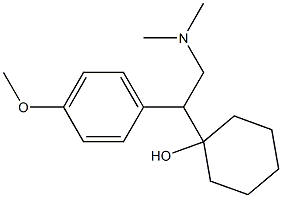
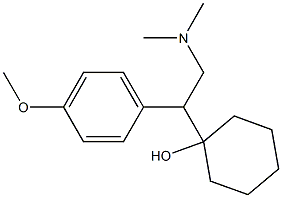
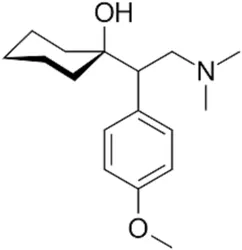
Venlafaxine hydrochloride
Synonym(s):(+/-)-1-[2-(Dimethylamino)-1-(4-methoxyphenyl)ethyl]cyclohexanol hydrochloride;Effexor;Venlafaxine hydrochloride
- CAS NO.:99300-78-4
- Empirical Formula: C17H28ClNO2
- Molecular Weight: 313.86
- MDL number: MFCD03658865
- EINECS: 619-421-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
What is Venlafaxine hydrochloride ?
Description
Venlafaxine hydrochloride, a novel phenethylamine derivative, was introduced in the U.S.A. as an antidepressant. Venlafaxine is reported to be the first in the class of the second-generation of antidepressants with dual serotonidnorepinephrine reuptake inhibitory activity. Venlafaxine lacks any affinity for muscarinic, cholinergic, histaminergic and noradrenergic receptors and therefore, has an unusually favorable side effect profile compared with classical tricyclic antidepressants and shows less cardiotoxicity. In addition, venlafaxine has a rapid onset of action that makes it unique among the antidepressant agents. Other indications for venlafaxine include the treatment of obsessive and panic disorders, and obesity. Both enantiomers of venlafaxine were reported to have similar biological activity.
Chemical properties
White Crystalline Powder
Originator
Wyeth-Ayerst (U.S.A.)
The Uses of Venlafaxine hydrochloride
Venlafaxine hydrochloride is an inhibitor of reuptake of both serotonin (IC50 = 0.21 μM) and norepinephrine (IC50 = 0.64 μM). It is effective in vitro and in vivo and against human as well as rat receptors. As an antidepressant, it is properly placed in the serotonin-norepinephrine reuptake inhibitor class.[Cayman Chemical]
The Uses of Venlafaxine hydrochloride
A selective serotonin noradrenaline reuptake inhibitor. Used as an antidepressant
The Uses of Venlafaxine hydrochloride
An inhibitor of ST and SLC6A2.
What are the applications of Application
Venlafaxine Hydrochloride is an inhibitor of ST and SLC6A2
Manufacturing Process
1-[Cyano(-methoxyphenyl)methyl]cyclohexanol
p-Methoxyphenylacetonitrile (50 gm, 0.3 mole) was added to dry
tetrahydrofuran (250 ml) and the solution cooled to -70°C under nitrogen. n-
Butyl lithium in hexane (210 ml, 0.3 mole) was added dropwise, with stirring.
The temperature was maintained below -50°C and a yellow precipitate
appeared. After the addition was complete, the reaction mixture was
maintained below -50°C for 30 minutes and cyclohexanone (35 ml, 0.3 mole)was added. After a further 45 minutes below -50°C the temperature was
allowed to rise to 0°C and a saturated ammonium chloride solution was
added. The layers were separated and the aqueous layer extracted with
diethyl ether. The combined organic solution was washed with brine, dried
over magnesium sulfate and evaporated. The product crystallized (25.2 gm,
melting point 125°-127°C). The structure was confirmed by N.M.R. and mass
spectral analysis.
1-[2-Amino-1-(p-methoxyphenyl)ethyl]cyclohexanol
1-[Cyano(p-methoxyphenyl)methyl]cyclohexanol (12 g, 0.05 mole) was
dissolved on warming in a mixture of ammonia-ethanol (20% v/v, 250 ml)
and hydrogenated in a Parr apparatus over 5% rhodium an alumina (2.8 gm).
The catalyst was filtered, washed well with ethanol and the combined filtrate
evaporated and dried under vacuum yielding an oil (12 gm). Thin layer
chromatography: single spot, ninhydrin positive [chloroform-methanol-acetic
acid (80:10:10 v/v)].
1-[-2-Dimethyl-amino)-1-(4-methoxyphenyl)-ethyl]cyclohexanol
1-[2-Amino-1-(p-methoxyphenyl)ethyl]cyclohexanol (12 gm; 0.048 mole) was
treated with a mixture of formaldehyde (11 ml), formic acid (14.5 ml, 88%)
and water (125 ml) and heated at 100°C for five hours. The reaction mixture
was cooled and extracted with ethyl acetate. This extract was discarded. The
aqueous residue was cooled in ice, rendered basic by the addition of solid
potassium hydroxide, saturated with sodium chloride and extracted 3 times
with ethyl acetate. The extract was washed with brine, dried over anhydrous
potassium carbonate and evaporated to an oily residue (8 gm). This mixture
of products was chromatographed on 1 kg of Mallinckrodt Silicar CC7 silica gel
and the progress of the chromatography was monitored by thin layer
chromatrography using a system comprising ethanol:2 N ammonia:ethyl
acetate:cyclohexane 45:8:100:100 (v/v). Fractions containing the desired
product were combined and the hydrochloride salt prepared using 4 N HCl in
isopropanol. The yield of the free base was 4.6 gm of 1-[(2-dimethylamino)-
1-(4-methoxyphenyl)ethyl]-cyclohexanol. The hydrochloride (venlafaxine):
melting point 215°-217°C. The structure was confirmed by mass spectral
analysis and N.M.R. analysis.
brand name
Effexor (Wyeth).
Therapeutic Function
Antidepressant
General Description
A Certified Snap-N-Spike? Solution suitable for many LC/MS and GC/MS applications from forensic or clinical toxicology analysis to urine drug testing. Also known by the brand name Effexor?, venlafaxine is an SNRI antidepressant approved for the treatment of major depressive and general anxiety disorders.
Biological Activity
Dual serotonin/noradrenalin re-uptake inhibitor that displays ~ 30-fold higher affinity for SERT than NET (K i values are 82 and 2480 nM respectively). Antidepressant; increases swimming and climbing behavior in the forced-swim test in rats.
Biochem/physiol Actions
Venlafaxine is an antidepressant. The mechanism of the antidepresant action of venlafaxine in humans is associated with its potentiation of neurotransmitter activity in the CNS. Venlafaxine is a potent inhibitor of neuronal serotonin and norepinephrine reuptake and weak inhibitor of dopamine reuptake. Venlafaxine has no significant activity for muscarinic, histaminergic, or α-1 adrenergic receptors in vitro. Venlafaxine does not possess MAO inhibitor activity.
Storage
Store at RT
Properties of Venlafaxine hydrochloride
| Melting point: | 207-209°C |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | H2O: >10mg/mL |
| form | powder |
| color | white |
| Merck | 14,9946 |
| CAS DataBase Reference | 99300-78-4(CAS DataBase Reference) |
| EPA Substance Registry System | Cyclohexanol, 1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl]-, hydrochloride (1:1 (99300-78-4) |
Safety information for Venlafaxine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P501:Dispose of contents/container to..… |
Computed Descriptors for Venlafaxine hydrochloride
| InChIKey | QYRYFNHXARDNFZ-UHFFFAOYSA-N |
Venlafaxine hydrochloride manufacturer
SRINI PHARMACEUTICALS PVT LTD
Besil Chem LLP
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![8-Azabicyclo[3.2.1]octane, 3-chloro-8-methyl-](https://img.chemicalbook.in/CAS/GIF/51275-31-1.gif)
You may like
-
 VENLAFAXINE 99300-78-4 95-99%View Details
VENLAFAXINE 99300-78-4 95-99%View Details
99300-78-4 -
 Venlafaxine HCl 98%View Details
Venlafaxine HCl 98%View Details -
 Venlafaxine HCl 98%View Details
Venlafaxine HCl 98%View Details -
 Venlafaxine CAS 99300-78-4View Details
Venlafaxine CAS 99300-78-4View Details
99300-78-4 -
 Venlafaxine Hcl Api, USPView Details
Venlafaxine Hcl Api, USPView Details
93413-69-5 -
 Venlafaxine Hcl Api, IPView Details
Venlafaxine Hcl Api, IPView Details
99300-78-4 -
 Venlafaxine HclView Details
Venlafaxine HclView Details
99300-78-4 -
 Venlafaxine HCL, THIRD PARTY MNFG, 25View Details
Venlafaxine HCL, THIRD PARTY MNFG, 25View Details
99300-78-4
