OXALYL BROMIDE
Synonym(s):OB;CAD11;cadherin 11, type 2, OB-cadherin (osteoblast);CDHOB;Ethanedioyl dibromide
- CAS NO.:15219-34-8
- Empirical Formula: C2Br2O2
- Molecular Weight: 215.83
- MDL number: MFCD00000113
- EINECS: 239-271-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-31 13:32:20
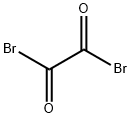
What is OXALYL BROMIDE?
Chemical properties
clear yellow to yellow-green liquid
The Uses of OXALYL BROMIDE
Used in synthetic applications of carbon-substituted iminium salts
Reactant for:
Synthesis of mutasynthons added to cultures of A. pretiosum for mutasynthetic generation of ansamitocin derivatives
Oxalic acid formation from hydroxyl radical substitutions
Cyclization to produce CRF1 receptor antagonists
Preparation, optical and electrochemical studies of thiophene end capped olig(2,3-alkylthieno[3,4-b]pyrazine)
Asymmetrical synthesis of glycosyl chlorides and bromides
The Uses of OXALYL BROMIDE
Leptin from rat has been used:
- to check the effect of intranasal nerve growth factor (NGF) on NGF activity in the spinal cord of Sprague-Dawley rats
- for validation of labelling for leptin-bearing cells of Sprague-Dawley rats
- to study the effect of leptin on STAT3 (signal transducer and activator of transcription 3) phosphorylation in neural population of Sprague-Dawley rats
- to study leptin response towards chemoreflex in nucleus of the solitary tract
- in rats, to check the effect of association between leptin and neuronal nitric oxide pathway on penicillin-induced epileptiform activity
Biochem/physiol Actions
Leptin is a hormone produced primarily in adipocytes, although leptin mRNA has also been identified in placenta and fetal tissues, gastric tissue and liver. Its primary site of action appears to be on neurons in the hypothalamus that are involved in regulating energy balance, appetite, and body weight. Leptin increases the production of nitric oxide in endothelial cells and stimulates angiogenesis in vitro and in vivo.Human and mouse leptin share ~84% sequence identity.
Properties of OXALYL BROMIDE
| Melting point: | -19 °C (lit.) |
| Boiling point: | 16-17 °C/10 mmHg (lit.) |
| Density | 1.517 g/mL at 25 °C |
| refractive index | n |
| Flash point: | -19°C |
| storage temp. | 2-8°C |
| solubility | Benzene (Slightly), Chloroform |
| form | Liquid |
| color | Clear |
| BRN | 1744437 |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 15219-34-8 |
| EPA Substance Registry System | Ethanedioyl dibromide (15219-34-8) |
Safety information for OXALYL BROMIDE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for OXALYL BROMIDE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 Oxalyl bromide CAS 15219-34-8View Details
Oxalyl bromide CAS 15219-34-8View Details
15219-34-8 -
 Oxalyl bromide CAS 15219-34-8View Details
Oxalyl bromide CAS 15219-34-8View Details
15219-34-8 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4