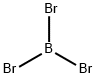
Aluminum Bromide
Synonym(s):Aluminium bromide;Aluminum tribromide;Aluminum tribromide (AlBr3 )
- CAS NO.:7727-15-3
- Empirical Formula: AlBr3
- Molecular Weight: 266.69
- MDL number: MFCD00003421
- EINECS: 231-779-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
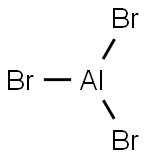
What is Aluminum Bromide?
Chemical properties
White Crystalline Powder
Physical properties
Colorless crystalline solid in anhydrous form; melts at 97.5°C; boils at 256°C;density 3.01 g/cm3 at 25°C; moisture sensitive, fumes in air; soluble in water (reacts violently in cold water, and decomposes in hot water, alcohols, acetone, hexane, benzene, nitrobenzene, carbon disulfide and many other organic sol_x0002_vents).
The Uses of Aluminum Bromide
Alkylation, bromination and isomerization catalyst in organic synthesis.Aluminum bromide acts as a strong Lewis acid and is used in Lewis acid-promoted reactions such as epoxide ring openings. It is used as a catalyst for Friedel-Crafts alkylation reaction. It also finds application in bromination and isomerization reactions in organic synthesis. It is also used in polymerization reaction to prepare poly(o-xylene)from benzocyclobutene.
The Uses of Aluminum Bromide
Anhydrous: bromination, alkylation, and isomerization catalyst in organic synthesis.
Preparation
Prepared from bromine and metallic aluminum.
2Al + 3Br2 ——? Al2Br6 (anhydrous).
General Description
A white to yellowish-red, lumpy solid with a pungent odor.
Reactivity Profile
An acid. May catalyze organic reactions. Corrosive to metals. Solutions of aluminum bromide in dichloromethane should be kept cold as a potentially dangerous exothermic halide exchange reaction occurs on warming, [Acc. Chem. Res., 1986, 19(3), 78].
Hazard
The anhydrous form reacts violently with water; corrosive to skin.
Health Hazard
CORROSIVE and/or TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire will produce irritating, corrosive and/or toxic gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
EXCEPT FOR ACETIC ANHYDRIDE (UN1715), THAT IS FLAMMABLE, some of these materials may burn, but none ignite readily. May ignite combustibles (wood, paper, oil, clothing, etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Flammable/toxic gases may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water. Substance may be transported in a molten form.
Safety Profile
A toxic, corrosive material. See also BROMIDES and ALUMINUM COMPOUNDS. Mixtures with sodium or potassium explode violently upon impact. When heated to decomposition it emits toxic fumes of Br-. Do not add H2O to anhydrous material. Hydrolysis can be violent.
Purification Methods
Reflux it and then distil it from pure aluminium chips in a stream of nitrogen into a flask containing more of the chips. It is then distilled under vacuum into ampoules [Tipper & Walker J Chem Soc 1352 1959]. Anhydrous conditions are essential, and the white to very light brown solid distillate can be broken into lumps in a dry-box (under nitrogen). It fumes in moist air. [Becher in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 812-813 1963.]
Properties of Aluminum Bromide
| Melting point: | 94-98 °C(lit.) |
| Boiling point: | 265 °C |
| Density | 3.205 g/mL at 25 °C(lit.) |
| vapor pressure | 1 mm Hg ( 81.3 °C) |
| Flash point: | 268°C subl. |
| storage temp. | 2-8°C |
| solubility | Soluble in benzene, nitrobenzene, toluene, xylene, ether, simple hydrocarbons, alcohol, carbon disulfide, ether. |
| form | powder |
| color | White to orange |
| Specific Gravity | 2.64 |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| Merck | 14,332 |
| Dielectric constant | 3.4(100℃) |
| Stability: | Stable, but reacts violently with water. Incompatible with aqueous solutions, alcohols, acids. |
| CAS DataBase Reference | 7727-15-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Aluminum tribromide(7727-15-3) |
| EPA Substance Registry System | Aluminum bromide (AlBr3) (7727-15-3) |
Safety information for Aluminum Bromide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H290:Corrosive to Metals H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Aluminum Bromide
| InChIKey | PQLAYKMGZDUDLQ-UHFFFAOYSA-K |
Aluminum Bromide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Aluminum bromide CAS 7727-15-3View Details
Aluminum bromide CAS 7727-15-3View Details
7727-15-3 -
 Aluminum bromide CAS 7727-15-3View Details
Aluminum bromide CAS 7727-15-3View Details
7727-15-3 -
 Aluminum bromide CAS 7727-15-3View Details
Aluminum bromide CAS 7727-15-3View Details
7727-15-3 -
 Aluminium bromide CAS 7727-15-3View Details
Aluminium bromide CAS 7727-15-3View Details
7727-15-3 -
 Aluminum Bromide Anhydrous CAS 7727-15-3View Details
Aluminum Bromide Anhydrous CAS 7727-15-3View Details
7727-15-3 -
 Aluminum bromide CAS 7727-15-3View Details
Aluminum bromide CAS 7727-15-3View Details
7727-15-3 -
 Aluminum bromide solution CAS 7727-15-3View Details
Aluminum bromide solution CAS 7727-15-3View Details
7727-15-3 -
 Aluminum bromide CAS 7727-15-3View Details
Aluminum bromide CAS 7727-15-3View Details
7727-15-3
