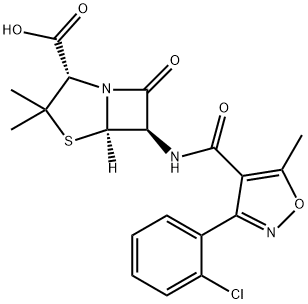
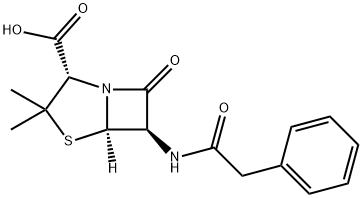
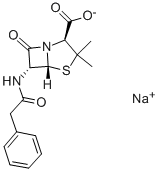
Oxacillin sodium monohydrate
Synonym(s):Oxacillin sodium salt monohydrate
- CAS NO.:7240-38-2
- Empirical Formula: C19H20N3NaO6S
- Molecular Weight: 441.43
- MDL number: MFCD00167146
- EINECS: 629-150-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22

What is Oxacillin sodium monohydrate?
Description
Oxacillin was synthesized by Bristol-Myers Laboratories in 1961 starting with 6-aminopenicillanic acid. It was the first orally active and penicillinase-stable semisynthetic penicillin to be introduced clinically. Oxacillin is slightly less stable against gastric acid and shows a lower serum concentration than phenoxymethylpenicillin, but it is highly active against phenoxymethylpenicillinresistant Staphylococcus aureus. Oxacillin has been used by oral and intramuscular administration for therapy of respiratory tract, urinary tract, gynecological, and other infections caused by benzylpenicillin-resistant bacteria.
Chemical properties
White or almost white powder.
Originator
Resistopen,Squibb,US,1962
The Uses of Oxacillin sodium monohydrate
Penicillin antibacterial.
The Uses of Oxacillin sodium monohydrate
Semi-synthetic antibiotic related to Penicillin. Antibacterial. Dyes and metabolites.
What are the applications of Application
Oxacillin Sodium Monohydrate is a member of the penicillin class of antibiotics
What are the applications of Application
Oxacillin Sodium Salt Monohydrate is a semi-synthetic antibiotic related to Penicillin
Definition
ChEBI: Oxacillin sodium monohydrate is a hydrate. It contains an oxacillin sodium.
Manufacturing Process
(A) Benzaldoxime: (Reference, Vogel, Textbook of Practical Organic Chemistry, page 883) -Materials: (Theoretical yield, 121.1 grams of free oxime), 106.1 grams (1.0 mol) of benzaldehyde (NF grade), 69.5 grams (1.0 mol) of hydroxylamine hydrochloride (practical grade), 68.0 grams (1.7 mol) of sodium hydroxide (pellet).
Procedure: The sodium hydroxide is dissolved in 200 ml water and the benzaldehyde is added. With continued stirring the hydroxylamine hydrochloride is added in portions. Some heat is developed and eventually the benzaldehyde dissolves. The solution is stirred for 15 minutes and then cooled in an ice-bath. A waxy, crystalline mass separates, and after further cooling it is collected by suction and dried in air. Yield is 86 to 149 grams. This crude material is suitable for step (B).
(B) Benzohydroximic Chloride: [Reference, G.W. Perrold et al, J. Am. Chem. Soc., 79, 462 (1957)] - Materials: 121 grams (0.77 mol) of crude benzaldoxime from step (A), 500 ml of 8.3 N hydrochloric acid, chlorine.
Procedure: The crude product from (A) is suspended in the hydrochloric acid, cooled in an ice-salt mixture, and chlorine is passed into the mixture with stirring for ? to 1 hour. Transient blue and green colors may be noticed in the mixture during this time. The temperature will probably rise to 3° to 5°C. The solid is collected by suction filtration and dried for an hour or so on the filter before use in (C). If at all possible, it should be used on the day of preparation. Yield is 71 grams (after 1? hours on the filter).
(C) 5-Methyl-3-Phenyl-4-Isoxazolecarboxylic Acid: [Reference, A. Quilico and R. Rusco, Gazz. Chim. Ital. 67, 589 (1937); C.A. 32, 21177] - Materials: 71 grams (0.45 mol) of crude benzohydroximic chloride from (E), 78 grams (0.60 mol) of ethyl acetoacetate (practical grade), 34 grams (0.60 mol) of sodium methoxide (95% minimum), 400 ml of methanol (reagent grade).
Procedure: The sodium methoxide is cautiously added in portions to 200 ml of methanol with stirring. Some heat is evolved. To this warm solution is rapidly added the ethyl acetoacetate with continued stirring. The solution is stirred for 10 minutes and then cooled in an ice-salt-acetone mixture (-25°C). If desired a Dry Ice-acetone cooling bath may be used to shorten the addition time. The crude material from (B) is dissolved in 200 ml of methanol. At this point it is probably easier to filter this mixture by suction to remove a large amount of insoluble solid, which is probably sodium chloride. The solid may be rinsed with more methanol.
The filtrate is chilled in ice-water and added to the cooled methanolic solution of the sodium derivative of ethyl acetoacetate at a rate which keeps the temperature of the re. action mixture below 0°C. The addition time will be 15 to 20 minutes if ice-salt-acetone is used as a coolant. This reaction is extremely exothermic.
The reaction mixture is stirred overnight at room temperature and filtered to remove the sodium chloride. The filtrate is stripped in vacuo and the crude ester (literature reports MP 48°C) is dissolved in 150 ml of ethanol; 28 grams (0.70 mol of sodium hydroxide in 90 ml of water is added and the solution is refluxed for 2 hours. After removal of the ethanol in vacuo the residue is dissolved in water and extracted twice with ether. Dissolved ether is removed from the aqueous solution in vacuo and it is acidified to pH 2 with concentrated hydrochloric acid.
The crystalline crude acid is dried briefly and then recrystallized from acetonitrile to give 32 grams of white product; MP 193° to 194.5°C (literature reports 189° to 190°C). Concentration of the mother liquor gives an additional 5 grams of material having a MP of 192.5 to 194°C. The 37 grams of material represents an 18% overall yield from benzaldehyde.
(D) The acid is converted to the acid chloride by reaction with thionyl chloride.
(E) 5-Methyl-3-Phenyl-4-Isoxazolylpenicillin: A solution of 4.43 grams of 5methyl-3-phenylisoxazole-4-carbonyl chloride in 120 ml acetone was added
gradually to a stirred solution of 4.32 grams of 6-aminopenicillanic acid in 168 ml of 3% aqueous sodium bicarbonate and 50 ml acetone. When addition was complete the mixture was stirred at room temperature for 4 hours and then extracted with ether (2 x 200 ml), only the aqueous phase being retained. This aqueous solution was covered with 50 ml ether and adjusted to pH 2 by the addition of N hydrochloric acid. After separating the layers, the aqueous phase was extracted with two further 50 ml portions of ether. The combined ether solutions (which at this stage contained the free penicillin acid) were washed with water and then neutralized by shaking with 20 ml N sodium bicarbonate solution. The aqueous phase was separated, washed with ether, and evaporated at low temperature and pressure to leave the crude sodium salt of 5-methyl-3-phenyl-4-isoxazolylpenicillin as a white solid, which was finally dried in vacuo over phosphorus pentoxide and found to weigh 7.34 grams.
brand name
Bactocill (GlaxoSmithKline); Prostaphlin (Apothecon).
Therapeutic Function
Antibacterial
Contact allergens
Oxacillin is a semisynthetic penicillin of the group M. It is closely related to cloxacillin.
Properties of Oxacillin sodium monohydrate
| Melting point: | 188 C |
| Boiling point: | 687℃ |
| alpha | D20 +201° (c = 1 in water) |
| refractive index | 205 ° (C=1, H2O) |
| Flash point: | >110°(230°F) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Freely soluble in water, soluble in methanol, practically insoluble in methylene chloride. |
| form | neat |
| form | Solid |
| color | White to Almost white |
| PH | pH (30g/l, 25℃) : 4.5~7.5 |
| Water Solubility | Freely soluble in water |
| Merck | 14,6904 |
| BRN | 4287093 |
| CAS DataBase Reference | 7240-38-2(CAS DataBase Reference) |
Safety information for Oxacillin sodium monohydrate
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. P405:Store locked up. |
Computed Descriptors for Oxacillin sodium monohydrate
Oxacillin sodium monohydrate manufacturer
Varanous Labs Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Oxacillin Sodium Salt Monohydrate CAS 7240-38-2View Details
Oxacillin Sodium Salt Monohydrate CAS 7240-38-2View Details
7240-38-2 -
 Oxacillin sodium monohydrate 95% CAS 7240-38-2View Details
Oxacillin sodium monohydrate 95% CAS 7240-38-2View Details
7240-38-2 -
 Oxacillin sodium salt monohydrate CAS 7240-38-2View Details
Oxacillin sodium salt monohydrate CAS 7240-38-2View Details
7240-38-2 -
 Oxacillin sodium CAS 7240-38-2View Details
Oxacillin sodium CAS 7240-38-2View Details
7240-38-2 -
 Oxacillin sodium CAS 7240-38-2View Details
Oxacillin sodium CAS 7240-38-2View Details
7240-38-2 -
 7240-38-2 Oxacillin Sodium 98%View Details
7240-38-2 Oxacillin Sodium 98%View Details
7240-38-2 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1