Osmium tetroxide
Synonym(s):‘Osmic acid’;Osmium(VIII)-oxide
- CAS NO.:20816-12-0
- Empirical Formula: O4Os
- Molecular Weight: 254.23
- MDL number: MFCD00011150
- EINECS: 244-058-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
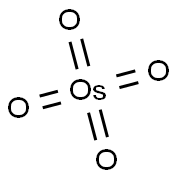
What is Osmium tetroxide?
Description
Osmium tetroxide (OsO4) is a highly toxic reagent that is typically used to make vicinal diols from alkenes. It is soluble in many organic solvents, only moderately soluble in water.
Chemical properties
solid with an unpleasant odour
Chemical properties
Osmium is a blue-white metal. It is found in platinum ores and in the naturally occurring alloy osmiridium. Osmium when heated in air or when the finely divided form is exposed to air at room temperature, oxidizes to form the tetroxide (OsO4), osmic acid. Osmium tetraoxide is a colorless, crystalline solid or pale-yellow mass. Unpleasant, acrid, chlorine-like odor. A liquid above 41°C.
Chemical properties
Osmium tetroxide is a strong oxidant. Numerous organic substances reduce it to black osmium dioxide (OsO2) or to osmium metal.
Physical properties
Pale, yellow crystalline solid; chlorine-like acrid odor; monoclinic crystals having terahedral structure; density 5.1 g/cm3; melts at 40.6°C; vaporizes at 129.7°C; sublimation begins below its boiling point; vapor pressure 11 torr at 27°C; critical temperature 405°C; critical pressure 170 atm; moderately soluble in water, 7.24 g/100mL at 25°C; soluble in most organic solvents.
The Uses of Osmium tetroxide
Osmium tetroxide is used in histopathological laboratories to stain the adipose tissue and as a stabilizing agent in scanning electron microscopy. In the chemical industry, it is used as a catalyst in the organic synthesis, particularly as the oxidizing agent in olefin-to-glycol conversion (546, 567). In the past, osmium tetroxide in the form of aqueous solution was used in forensic medicine to examine fingerprints. Osmium tetroxide is also used in medicine to treat rheumatoid arthritis.
The Uses of Osmium tetroxide

To a solution of the SM (1.94 g, 10.58 mmol) in a mixture of acetone (63 mL) and H2O (7.8 mL) at RT was added NMO (2.48 g, 21.16 mmol). After 2 min, OsO4 (4% in H2O, 3.37 mL) was added and the rxn mixture was stirred at RT for 23 h. The mixture was quenched with aq 0.2M Na2S2O3 (25 mL) and extracted with DCM (2 x 50 mL). The combined organics were washed with aq 0.2M Na2S2O3 (10 mL), dried (MgSO4), and concentrated in vacuo. The crude material was purified by flash chromatography (17-100% EtOAc/heptane) to provide the product as a yellow solid. [1.31 g, 57%]
The Uses of Osmium tetroxide
Oxidizing agent, particularly for converting olefins to glycols. Catalyzes chlorate, peroxide, periodate, and other oxidations: P. N. Rylander, Organic Syntheses with Noble Metal Catalysts (Academic Press, New York, 1973) pp 121-144. As fixing and staining agent for cell and tissue studies.
The Uses of Osmium tetroxide
Osmium (VIII) tetraoxide (Os8+ + 4O2-→ OsO4) is a yellow crystal and probably the most important compound used as an oxidizing agent, as a biological stain in microscopy, and to detect fingerprints.
What are the applications of Application
Osmium tetroxide is a hydroxyl-donating compound for chemical synthesis
Definition
ChEBI: An osmium coordination entity consisting of four oxygen atoms bound to a central osmium atom via covalent double bonds.
Production Methods
Osmium tetroxide is obtained by heating, at 300–400°C, finely divided osmium metal in the stream of air or oxygen (546). Commercially, it is received during osmium smelting and platinum annealing. Osmium tetroxide may also be produced by oxidizing osmium with aqua regia or nitric acid. It is often formed at room temperature from osmium metal powder.
Preparation
Osmium tetroxide is obtained as an intermediate during recovery of osmium metal from osmiridium or other noble metal minerals (See Osmium). In general, oxidation of an aqueous solution of an osmium salt or complex, such as sodium osmate with nitric acid, yields the volatile tetroxide which may be distilled out from the solution. In the laboratory, the compound can be prepared by oxidation of the osmium tetrachloride, OsCl4, or other halide solutions with sodium hypochlorite followed by distillation.
Osmium tetroxide may also be produced by heating finely divided osmium metal in a stream of oxygen or air at 300 to 400°C.
General Description
A colorless or yellow solid with a pungent odor of chlorine. Melting point about 104°F. Boiling point 266°F (begins to sublime below melting point). Density 4.9 g / cm3. Soluble in alcohol. Toxic by inhalation and a strong irritant to the eyes and mucous membranes.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Osmium tetraoxide is incompatible with hydrochloric acid andeasily oxidized organic materials. Contact with other materials may cause fire. . Reacted explosively with1-methylimidazole [J. Chem. Soc., Dalton Trans., 1979, 1084].
Hazard
Osmium tetroxide is poisonous by all routes of exposure. The vapor is an eye irritant and can produce tears and damage. The vapor also can cause upper respiratory tract irritation.
LD50 oral (mouse): 162 mg/kg
LCLO inhalation (mouse): 40 ppm (104 mg/m 3)/4 hr.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Health Hazard
The acute toxicity of osmium tetroxide is high, and it is a severe irritant of the eyes and
respiratory tract. Exposure to osmium tetroxide vapor can damage the cornea of the eye.
Irritation is generally the initial symptom of exposure to low concentrations of osmium tetroxide
vapor, and lacrimation, a gritty feeling in the eyes, and the appearance of rings around lights may
also be noted. In most cases, recovery occurs in a few days. Concentrations of vapor that do not
cause immediate irritation can have an insidious cumulative effect; symptoms may not be noted
until several hours after exposure. Contact of the eyes with concentrated solutions of this
substance can cause severe damage and possible blindness. Inhalation can cause headache,
coughing, dizziness, lung damage, and difficult breathing and may be fatal. Contact of the vapor
with skin can cause dermatitis, and direct contact with the solid can lead to severe irritation and
burns. Exposure to osmium tetroxide via inhalation, skin contact, or ingestion can lead to
systemic toxic effects involving liver and kidney damage. Osmium tetroxide is regarded as a
substance with poor warning properties.
Chronic exposure to osmium tetroxide can result in an accumulation of osmium compounds in
the liver and kidney and damage to these organs. Osmium tetroxide has been reported to cause
reproductive toxicity in animals; this substance has not been shown to be carcinogenic or to show
reproductive or developmental toxicity in humans.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Fire Hazard
Noncombustible
Flammability and Explosibility
Noncombustible
Safety Profile
Poison by ingestion, inhalation, and intraperitoneal routes. Human systemic effects by inhalation: lachrymation and other eye effects and structural or functional changes in trachea or bronch. Experimental reproductive effects. Mutation data reported. Explodes on contact with 1 -methylimidazole. Catalytic decomposition of hydrogen peroxide can be hazardous. See also OSMIUM
Potential Exposure
Osmium may be alloyed with platinum metals, iron, cobalt, and nickel; and it forms compounds withtin and zinc. The alloy with iridium is used in the manufacture of fountain pen points, engraving tool; record player needles; electrical contacts; compass needles; fine machine bearings; and parts for watch and lock mechanisms. The metal is a catalyst in the synthesis of ammonia; and in the dehydrogenation of organic compounds. It is also used as a stain for histological examination of tissues. Osmium tetroxide is used as an oxidizing agent, catalyst, and as a fixative for tissues in electron microscopy. Other osmium compounds find use in photography. Osmium no longer is used in incandescent lights or in fingerprinting.
storage
In particular, all work with osmium tetroxide should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Osmium tetroxide as solid or solutions should be stored in tightly sealed containers, and these should be placed in secondary containers.
Purification Methods
It is VERY TOXIC and should be manipulated in a very efficient fume cupboard. It attacks the eyes severely (use also face protection) and is a good oxidising agent. It is volatile and has a high vapour pressure (11mm) at room temperature. It sublimes and volatilises well below its boiling point. It is soluble in *C6H6, H2O (7.24% at 25o), CCl4 (375% at 25o), EtOH and Et2O. It is estimated by dissolving a sample in a glass-stoppered flask containing 25mL of a solution of KI (previously saturated with CO2) and acidified with 0.35M HCl. After gentle shaking in the dark for 30minutes, the solution is diluted to 200mL with distilled H2O saturated with CO2 and titrated with standard thiosulfate using starch as indicator. This method is not as good as the gravimetric method. Hydrazine hydrochloride (0.1 to 0.3g) is dissolved in 3M HCl (10mL) in a glass-stoppered bottle. After warming to 55-65o, a weighed sample of OsO4 solution is introduced, and the mixture is digested on a water bath for 1hour. The mixture is transferred to a weighed glazed crucible and evaporated to dryness on a hot plate. A stream of H2 is started through the crucible, and the crucible is heated over a burner for 20-30minutes. The stream of H2 is continued until the crucible in cooled to room temperature, and then the H2 is displaced by CO2 in order to avoid rapid combustion of H2. Finally the crucible is weighed. [Grube in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II pp 1603 1965, Anderson & Yost J Am Chem Soc 60 1822 1938.] § Available commercially on a polymer support.
Incompatibilities
Osmium tetroxide is a strong oxidizer. Reacts with combustibles and reducing materials. Reacts with hydrochloric acid to form toxic chlorine gas. Forms unstable compounds with alkalis.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of Osmium tetroxide
| Melting point: | 40°C |
| Boiling point: | 130 °C |
| Density | 1.04 |
| vapor density | 8.8 (vs air) |
| vapor pressure | 7 mmHg at 20 °C |
| Flash point: | -40 °C |
| storage temp. | 2-8°C |
| solubility | Soluble in alcohol, ether, chloroform, benzene, ammonium hydroxide, phosphorus oxychloride and carbon tetrachloride |
| form | Solution |
| appearance | Pale yellow solid (sublimes at room temperature) |
| color | White to yellow,or lemon yellow |
| Specific Gravity | 5.10 |
| Odor | Acrid, chlorine-like odor detectable at 2 ppm (20 mg/m3) |
| Water Solubility | Soluble in chloroform, alcohol and ethers.Soluble in water, organic solvents, benzene, alcohol, ether, ammonium hydroxide, phosphorus oxychloride and carbon tetrachloride. |
| Sensitive | Air Sensitive |
| Merck | 14,6893 |
| Stability: | Stable. Incompatible with strong acids, hydrogen chloride, organic materials, finely powdered metals. |
| CAS DataBase Reference | 20816-12-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Osmium tetraoxide(20816-12-0) |
| EPA Substance Registry System | Osmium tetroxide (20816-12-0) |
Safety information for Osmium tetroxide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Osmium tetroxide
Osmium tetroxide manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 Osmium(VIII) oxide CAS 20816-12-0View Details
Osmium(VIII) oxide CAS 20816-12-0View Details
20816-12-0 -
 Osmium(VIII) oxide CAS 20816-12-0View Details
Osmium(VIII) oxide CAS 20816-12-0View Details
20816-12-0 -
 Osmium(VIII) oxide CAS 20816-12-0View Details
Osmium(VIII) oxide CAS 20816-12-0View Details
20816-12-0 -
 Osmic Acid extrapure CAS 20816-12-0View Details
Osmic Acid extrapure CAS 20816-12-0View Details
20816-12-0 -
 Osmic acid, 99.9% CAS 20816-12-0View Details
Osmic acid, 99.9% CAS 20816-12-0View Details
20816-12-0 -
 Osmic acid, solution, 2% w/v CAS 20816-12-0View Details
Osmic acid, solution, 2% w/v CAS 20816-12-0View Details
20816-12-0 -
 OSMIUM TETROXIDE 2.5% SOLUTION IN TERT BUTANOL CAS 20816-12-0View Details
OSMIUM TETROXIDE 2.5% SOLUTION IN TERT BUTANOL CAS 20816-12-0View Details
20816-12-0 -
 Osmium tetraoxide 1 gm Sealed Ampoules 99% CAS 20816-12-0View Details
Osmium tetraoxide 1 gm Sealed Ampoules 99% CAS 20816-12-0View Details
20816-12-0
