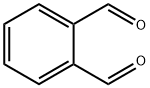
Phthalic anhydride
Synonym(s):Phthalic anhydride
- CAS NO.:85-44-9
- Empirical Formula: C8H4O3
- Molecular Weight: 148.12
- MDL number: MFCD00005918
- EINECS: 201-607-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:04
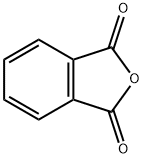
What is Phthalic anhydride?
Description
Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. This colourless solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics.

Phthalic anhydride is an important chemical intermediate in the plastics industry from which are derived numerous phthalate esters that function as plasticizers in synthetic resins. Phthalic anhydride itself is used as a monomer for synthetic resins such as glyptal, the alkyd resins, and the polyester resins.
Phthalic anhydride is also used as a precursor of anthraquinone, phthalein, rhodamine, phthalocyanine, fluorescein, and xanthene dyes.
Phthalic anhydride is used in the synthesis of primary amines, the agricultural fungicide phaltan, and thalidomide. Other reactions with phthalic anhydride yield phenolphthalein, benzoic acid, phthalylsulfathiazole (an intestinal antimicrobial agent), and orthophthalic acid.
Chemical properties
Phthalic Anhydride is moderately flammable, white solid (flake) or a clear, colorless, mobile liquid (molten) Characteristic, acrid, choking odor. It is very slightly soluble in H2O, soluble in alcohol, and slightly soluble in ether.
Physical properties
Colorless to pale cream crystals with a characteristic, choking odor. Moisture sensitive. Odor threshold concentration is 53 ppb (quoted, Amoore and Hautala, 1983).
The Uses of Phthalic anhydride
Phthalic anhydride is used in the manufacture of unsaturated polyesters and as a curing agent for epoxy resins. When used as a pigment, it can be responsible for sensitization in ceramic workers.
Definition
ChEBI: Phthalic anhydride is the cyclic dicarboxylic anhydride that is the anhydride of phthalic acid. It has a role as an allergen. It is a cyclic dicarboxylic anhydride and a member of 2-benzofurans.
Preparation
The most important modifying component used in the
manufacture of linear unsaturated polyesters is phthalic anhydride. The
anhydride is generally obtained by the oxidation of o-xylene:
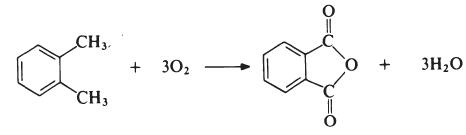
The reaction is carried out in the vapour phase by passing a mixture of o-xylene and air over a catalyst such as vanadium pentoxide supported on silica and promoted with titanium dioxide at about 400??C. The exit gases are cooled and the phthalic anhydride is collected and purified by distillation under reduced pressure.
Synthesis Reference(s)
The Journal of Organic Chemistry, 25, p. 616, 1960 DOI: 10.1021/jo01074a035
Synthesis, p. 612, 1973
Tetrahedron Letters, 20, p. 2301, 1979 DOI: 10.1016/S0040-4039(01)93957-7
General Description
A colorless to white lustrous solid in the form of needles with a mild distinctive odor. Moderately toxic by inhalation or ingestion and a skin irritant. Melting point 64°F Flash point 305°F. Forms a corrosive solution when mixed with water. Used in the manufacture of materials such as artificial resins.
Air & Water Reactions
Reacts, usually slowly with water to form phthalic acid and heat [Merck 11th ed. 1989]. The phthalic acid is somewhat soluble in water.
Reactivity Profile
Phthalic anhydride reacts exothermically with water. The reactions are sometimes slow, but can become violent when local heating accelerates their rate. Acids accelerate the reaction with water. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases. Undergoes exothmeric nitration with fuming nitric acid-sulfuric acid and may give mixtures of the potentially explosive phthaloyl nitrates or nitrites or their nitro derivatives [Chem. & Ind. 20:790. 1972]. Phthalic anhydride reacts violently with CuO at elevated temperatures [Park, Chang-Man, Richard J. Sheehan. hthalic Acids and Other Benzenepolycarboxylic Acids Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 2005]. Mixtures of Phthalic anhydride and anhydrous CO2 explode violently if heated [eaflet No. 5, Inst. of Chem., London, 1940].
Health Hazard
Solid irritates skin and eyes, causing coughing and sneezing. Liquid causes severe thermal burns.
Fire Hazard
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Pharmaceutical Applications
Phthalic anhydride reacted with cellulose acetate forms cellulose acetate phthalate (CAP), a common enteric coating excipient that has also been shown to have antiviral activity. Phthalic anhydride is a degradation product of CAP.
Contact allergens
Phthalic anhydride is used in the manufacture of unsaturated polyesters and as a curing agent for epoxy resins. When used as a pigment, it can be responsible for sensitization in ceramic workers. Phthalic anhydride per se is not responsible for the sensitization to the resin used in nail varnishes phthalic anhydride/trimellitic anhydride/ glycols copolymer.
Safety Profile
Poison by ingestion. Experimental teratogenic effects. A corrosive eye, skin , and mucous membrane irritant. A common air contaminant. Combustible when exposed to heat or flame; can react with oxidzing materials. Moderate explosion hazard in the form of dust when exposed to flame. The production of ths material has caused many industrial explosions. Mixtures with copper oxide or sodium nitrite explode when heated. Violent reaction with nitric acid + sulfuric acid above 80℃. To fight fire, use CO2, dry chemical. Used in plasticizers, polyester resins, and alkyd resins, dyes, and drugs. See also ANHYDRIDES.
Synthesis
Phthalic anhydride is a precursor to a variety of reagents useful in organic synthesis. Important derivatives include phthalimide and its many derivatives. Chiral alcohols form half-esters (see above), and these derivatives are often resolvable because they form diastereomeric salts with chiral amines such as brucine. A related ring - opening reaction involves peroxides to give the useful peroxy acid:
C6H4(CO)2O + H2O2 → C6H4(CO3H)CO2H.
Potential Exposure
Phthalic anhydride is used in plasticizers; in the manufacture of phthaleins; benzoic acid; alkyd and polyester resins; synthetic indigo; and phthalic acid;which is used as a plasticizer for vinyl resins. To a lesser extent, it is used in the production of alizarin, dye, anthranilic acid; anthraquinone, diethyl phthalate; dimethyl phthalate; erythrosine, isophthalic acid; methylaniline, phenolphthalein, phthalamide, sulfathalidine, and terephthalic acid. It has also found uses as a pesticide intermediate.
Shipping
UN2214 Phthalic anhydride with>.05 % maleic anhydride, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Distil the anhydride under reduced pressure. Purify it from the acid by extracting with hot CHCl3, filtering and evaporating. The residue is crystallised from CHCl3, CCl4 or *benzene, or sublimed. Fractionally crystallise it from its melt. Dry it under vacuum at 100o. [Saltiel J Am Chem Soc 108 2674 1986, Beilstein 17/11 V 253.]
Toxicity evaluation
Phthalic anhydride modulates lipid mediator release and cytokine formation and has sensitizing effects on the respiratory tract. The local irritating effect particularly on the mucous membranes probably depends on the hydrolysis to phthalic acid.
Incompatibilities
Dust forms an explosive mixture with air. Phthalic anhydride reacts exothermically with water. The reactions are sometimes slow, but can become violent when local heating accelerates their rate. Acids accelerate the reaction with water. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases. Converted to phthalic acid in hot water. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. caustics, ammonia, amines, water. Reacts violently with copper oxide or sodium nitrite 1 heat.
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of Phthalic anhydride
| Melting point: | 131-134 °C(lit.) |
| Boiling point: | 284 °C(lit.) |
| Density | 1,53 g/cm3 |
| vapor density | 5.1 (vs air) |
| vapor pressure | <0.01 mm Hg ( 20 °C) |
| refractive index | 1.4500 (estimate) |
| Flash point: | 152 °C |
| storage temp. | Store below +30°C. |
| solubility | 6g/l (slow decomposition) |
| form | Flaky Crystals |
| pka | 2.97[at 20 ℃] |
| color | White |
| Odor | Characteristic choking odor |
| PH | 2 (6g/l, H2O, 20℃) |
| PH Range | 2 at 6 g/l at 20 °C |
| explosive limit | 1.7-10.5%(V) |
| Water Solubility | 6 g/L (20 ºC) |
| Sensitive | Moisture Sensitive |
| Merck | 14,7372 |
| BRN | 118515 |
| Henry's Law Constant | 6.29 at 20 °C (approximate - calculated from water solubility and vapor pressure) |
| Exposure limits | NIOSH REL: TWA 6 mg/m3 (1 ppm), IDLH 60 mg/m3; OSHA PEL: TWA 12
mg/m3 (2 ppm); ACGIH TLV: TWA 1 ppm (adopted). |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong bases, moisture, strong acids. Dust may form an explosive mixture with air. |
| CAS DataBase Reference | 85-44-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Phthalic anhydride(85-44-9) |
| EPA Substance Registry System | Phthalic anhydride (85-44-9) |
Safety information for Phthalic anhydride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Phthalic anhydride
Phthalic anhydride manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Phthalic Anhydride 99%View Details
Phthalic Anhydride 99%View Details -
 PHTHALIC ANHYDRIDE 99%View Details
PHTHALIC ANHYDRIDE 99%View Details -
 Phthalic anhydride CAS 85-44-9View Details
Phthalic anhydride CAS 85-44-9View Details
85-44-9 -
 Phthalic Anhydride CASView Details
Phthalic Anhydride CASView Details -
 Phthalic Anhydride ACS CAS 85-44-9View Details
Phthalic Anhydride ACS CAS 85-44-9View Details
85-44-9 -
 Phthalic Anhydride 85-44-9View Details
Phthalic Anhydride 85-44-9View Details
85-44-9 -
 25 Kg Phthalic AnhydrideView Details
25 Kg Phthalic AnhydrideView Details
85-44-9 -
 Phthalic Anhydride PowderView Details
Phthalic Anhydride PowderView Details
85-44-9
