Di-tert-butyl dicarbonate
Synonym(s):Boc anhydride;Boc2O;Di-tert-butyl pyrocarbonate;Di-tert-butyl dicarbonate;Di-tert-butyl dicarbonate, Di-tert-butyl pyrocarbonate
- CAS NO.:24424-99-5
- Empirical Formula: C10H18O5
- Molecular Weight: 218.25
- MDL number: MFCD00008805
- EINECS: 246-240-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
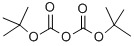
What is Di-tert-butyl dicarbonate?
Description
Boc anhydride has a melting point of only 22-24 C. It often starts to melt while being weighed out for a reaction. In order to use boc anhydride as a solid, it should be kept in a refrigerator and weighed out soon after being removed from the refrigerator.
Chemical properties
Di-tert-butyl dicarbonate (BOC Anhydride, DiBOC) is a colorless to white to yellow crystals, solidified mass or clear liquid. It melts around room temperature (m.p.=23°C). It does not decompose at this or even slightly higher temperatures. For example, it is typically purified by distillation under reduced pressure at temperatures up to around 65°C. At higher temperatures it will decompose to isobutene, t-butyl alcohol and carbon dioxide.
The Uses of Di-tert-butyl dicarbonate
Di-tert-butyl dicarbonate (Boc2O) is a widely used reagent for introducing protecting groups in peptide synthesis. It plays an important role in the preparation of 6-acetyl-1,2,3,4-tetrahydropyridine by reacting with 2-piperidone. It serves as a protecting group used in solid phase peptide synthesis.
The Uses of Di-tert-butyl dicarbonate

To a solution of the SM (1 g, 2.23 mmol) and TEA (1.24 mL, 8.93 mmol) in DCM (20 mL) under N2 was added (Boc)2O (1.46 g, 6.7 mmol) at 5-10 C. The reaction mixture was stirred overnight at RT, after which time it was diluted with H2O (20 mL) and extracted with DCM (2 x 20 mL). The combined org extracts were washed with H2O (2 x 20 mL), brine, dried (Na2SO4), and concentrated to provide the product as a white solid. [1.2 g, 83%]
Preparation
The preparation of Di-tert-butyl dicarbonate is as follows:To a monoester sodium salt solution were added 2g of N, N-dimethylformamide, 1g of pyridine, 1g of triethylamine,Cooling to -5~0°C, 60g diphosgene was slowly added dropwise within 1.5h dropwise addition was complete, warmed to room temperature (25°C), incubated for 2h, the reaction was allowed to stand after filtration, washing organic solution. Dried with anhydrous magnesium sulfate, the solvent was distilled off at atmospheric pressure to give crude product 65~70g. After cooling and crystallization, 57-60g of di-tert-butyl dicarbonate were obtained in a yield of 60-63%.
Definition
ChEBI: Di-tert-butyl dicarbonate is an acyclic carboxylic anhydride. It is functionally related to a dicarbonic acid.
What are the applications of Application
Di-tert-butyl dicarbonate has the following uses:
(1) As part of a series of bovine plasma amine oxidase inactivators. Aminomethylenes were prepared by the reaction of Boc propargylamine with formaldehyde, diisopropylamine and copper bromide.
(2) It can be used as a general purpose carboxylation reagent. Carbon nucleophiles generated by a non-nucleophilic base (LDA) were effectively trapped with di-tert-butyl dicarbonate (Boc-anhydride) to provide the corresponding tert-butyl aryl acetates, di-tert-butyl aryl malonates, unsymmetrical aryl malonates and tert-butyl benzoates in high yields.
(3) Alcohols as Boc derivatives were catalytically protected by Lewis acids. Reagents for the introduction of Boc protecting groups.
(4) Reagents for the preparation of Boc-protected amines. Tri-tert-butoxycarbonyl-protected hydrazines prepare Fmoc esters in chromogenic reagents for monitoring solid-phase aldehydes.
Reactions
The reaction of substituted anilines with Boc2O in the presence of a stoichiometric amount of 4-dimethylaminopyridine (DMAP) in an inert solvent (acetonitrile, dichloromethane, ethyl acetate, tetrahydrofuran, toluene) at room temperature leads to aryl isocyanates in almost quantitative yields within 10 min.
Di-tert-butyl dicarbonate and 4-(dimethylamino)pyridine revisited. Their reactions with amines and alcohols
General Description
Di-tert-butyl dicarbonate (Boc2O) is a reagent mainly used for the introduction of the Boc protecting group to amine functionalities. It is also used as a dehydrating agent in some organic reactions, particularly with carboxylic acids, certain hydroxyl groups, or with primary nitroalkanes.
Hazard
An irritant that may cause serious eye injury; May cause skin sensitization; Highly toxic by inhalation
Flammability and Explosibility
Flammable
Purification Methods
Melt the ester by heating at ~35o, and distil it in a vacuum. If IR and NMR ( 1810m 1765 cm-1 , in CCl4 1.50 singlet) suggest very max impure, then wash with an equal volume of H2O containing citric acid to make the aqueous layer slightly acidic, collect the organic layer and dry it over anhydrous MgSO4 and distil it in a vacuum. [Pope et al. Org Synth 57 45 1977, Keller et al. Org Synth 63 160 1985, Grehn et al. Angew Chem 97 519 1985.] FLAMMABLE.
Properties of Di-tert-butyl dicarbonate
| Melting point: | 23 °C (lit.) |
| Boiling point: | 56-57 °C/0.5 mmHg (lit.) |
| Density | 0.95 g/mL at 25 °C (lit.) |
| vapor pressure | 3.85Pa at 25℃ |
| refractive index | n |
| Flash point: | 99 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Sparingly), Methanol (Slightly) |
| form | Low Melting Crystalline Solid |
| appearance | Colorless solid |
| color | White |
| Specific Gravity | 0.950 |
| Water Solubility | Miscible with decalin, toluene, carbon tetrachloride, tetrahydrofuran, dioxane, alcohols, acetone, acetonitrile and dimethylformamide. Immiscible with water. |
| Sensitive | Moisture Sensitive |
| BRN | 1911173 |
| Stability: | Acid Sensitive |
| CAS DataBase Reference | 24424-99-5(CAS DataBase Reference) |
| EPA Substance Registry System | Dicarbonic acid, bis(1,1-dimethylethyl) ester (24424-99-5) |
Safety information for Di-tert-butyl dicarbonate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Di-tert-butyl dicarbonate
| InChIKey | DYHSDKLCOJIUFX-UHFFFAOYSA-N |
Di-tert-butyl dicarbonate manufacturer
Lanya Chem Industries Pvt Ltd
Cocreate Global Technologies Pvt Ltd
Arown Pharma
Chemfield Inc
ASM Organics
Related products of tetrahydrofuran


You may like
-
 24424-99-5 98%View Details
24424-99-5 98%View Details
24424-99-5 -
 Di-tert-butyl dicarbonate 98%View Details
Di-tert-butyl dicarbonate 98%View Details -
 Di-tert-Butyldicarbonate (BOC Anhydride CAS 24424-99-5View Details
Di-tert-Butyldicarbonate (BOC Anhydride CAS 24424-99-5View Details
24424-99-5 -
 Boc-anhydride CAS 24424-99-5View Details
Boc-anhydride CAS 24424-99-5View Details
24424-99-5 -
 Boc anhydride CAS 24424-99-5View Details
Boc anhydride CAS 24424-99-5View Details
24424-99-5 -
 Di-Tert-Butyl Dicarbonate CASView Details
Di-Tert-Butyl Dicarbonate CASView Details -
 Di-Tert-Butyl Dicarbonate CASView Details
Di-Tert-Butyl Dicarbonate CASView Details -
 Di-Tert-Butyl Dicarbonate CASView Details
Di-Tert-Butyl Dicarbonate CASView Details