ORITAVANCIN
- CAS NO.:171099-57-3
- Empirical Formula: C86H97Cl3N10O26
- Molecular Weight: 1793.1
- MDL number: MFCD12756396
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-25 20:05:57
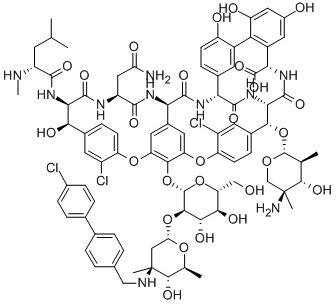
What is ORITAVANCIN?
Absorption
Pharmacokinetic analysis of oritavancin revealed a Cmax of 138 and μg/mL and an AUC0-∞ of 2800 μg?h/mL. The AUC0-t in a study of healthy volunteers after an 800 mg dose 1,1111 μg?h/mL. was also be Another pharmacokinetic study reported a Cmax of 4.7-7.6 micrograms/mL, generally achieved within 24 hours of administration.
Toxicity
The LD50 of oritavancin in rats is >500m mg/kg. Prescribing information indicates no experience with overdose during the clinical program for oritavancin, however, an overdose is likely to result in an increased risk of adverse effects, such as headache, nausea vomiting, and diarrhea. This drug is not dialyzable, and in the case of an overdose, supportive measures should be undertaken.
Indications
Oritavancin is indicated for the treatment of adult patients with acute bacterial skin and skin structure (including subcutaneous) infection. It is used for confirmed/suspected infections with designated and susceptible gram-positive organisms. There are two preparations of oritavancin; the 400 mg dose that is administered over 3 hours, and the 1200 mg dose administered over 1 hour. Both are indicated for susceptible gram-positive skin and skin structure infections in adults.
As antimicrobial susceptibility patterns are geographically distinct, local antibiograms should be consulted to ensure adequate coverage of relevant pathogens prior to use.
Background
Oritavancin is a glycopeptide antibiotic used for the treatment of skin infections. It was developed by The Medicines Company (acquired by Novartis). Oritavancin was initially approved by the FDA in 2014 and formulated to combat susceptible gram-positive bacteria that cause skin and skin structure infections. It boasts the option of single-dose administration and has been proven as non-inferior to a full course of vancomycin therapy.
On March 12, 2021 the FDA approved Kimyrsa, a complete course of therapy in a single, 1 hour 1200 mg infusion. Orbactiv, the other FDA approved oritavancin product, is administered over a 3 hour infusion and contains a lower dose of 400 mg. Marketed by Melinta Therapeutics, Kimyrsa offers effective and time-efficient treatment for skin and skin structure infections.
Definition
ChEBI: A semisynthetic glycopeptide used (as its bisphosphate salt) for the treatment of acute bacterial skin and skin structure infections caused or suspected to be caused by susceptible isolates of designated Gram-positive microorganisms including MRSA.
Pharmacokinetics
Oritavancin interferes with bacterial cell wall synthesis and integrity, treating susceptible skin and subcutaneous tissue infections with gram-positive bacteria. This drug is known to artifically increase INR and aPTT, interfering with coagulation testing. Cases of infusion reactions have also been reported.
Clinical Use
Antibacterial agent
Enzyme inhibitor
This novel, semi-synthetic glycopeptide antibiotic (FW = 1793.10 g/mol; CAS 171099-57-3), also known as LY333328, Orbactiv?, and (4R)-22-O- (3-amino-2,3,6-trideoxy-3-C-methyl-a-L-arabinohexopyranosyl)-N3-(p-(pchlorophenyl) benzyl)vancomycin, disrupts the cell membrane of Grampositive bacteria and inhibits both transglycosylation and transpeptidation. Oritavancin differs from vancomycin by the presence of a hydrophobic N-4-(4-chlorophenyl)benzyl substituent on the disaccharide, the addition of a 4-epi-vancosamine monosaccharide to the amino acid residue in Ring-6, and replacement of the vancosamine moiety by 4-epi-vancosamine. When compared vancomycin, teicoplanin, and quinupristin-dalfopristin (Synercid) against 219 strains of enterococci and staphylococci, including vancomycin-resistant enterococci and methicillinresistant methicillinresistant Staphylococcus aureus, LY333328 demonstrated superior activity against vancomycin-resistant enterococci and was the only antibiotic which was bactericidal. Indeed, a single infusion of this antibiotic (1200 mg) can clear serious bacterial skin infections, including methicillin-resistant Staphylococcus aureus, or MRSA, as effectively as the usual 7-10 day, twice-daily regimen of vancomycin now needed to treat patients.
Drug interactions
Potentially hazardous interactions with other drugs
Anticoagulants: possibly increases warfarin
concentration.
Metabolism
In vitro studies on human hepatocytes suggest that oritavancin is not metabolized, and is excreted unchanged.
Metabolism
In vitro human liver microsome studies indicated that oritavancin is not metabolised. It is excreted unchanged; less than 1% and 5% of a dose is recovered in the urine and the faeces.
Properties of ORITAVANCIN
| Density | 1.59±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | 2.93±0.70(Predicted) |
Safety information for ORITAVANCIN
Computed Descriptors for ORITAVANCIN
ORITAVANCIN manufacturer
Sumar biotech LLP
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran



![[4-(4-CHLOROPHENYL)PHENYL]METHYLAMINE HYDROCHLORIDE](https://img.chemicalbook.in/CAS/GIF/410077-96-2.gif)
You may like
-
 171099-57-3 Oritavancin 98%View Details
171099-57-3 Oritavancin 98%View Details
171099-57-3 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Phenylephrine HCl 61-76-7 98%View Details
Phenylephrine HCl 61-76-7 98%View Details
61-76-7 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3 -
 Abiretorone 154229-18-2 98%View Details
Abiretorone 154229-18-2 98%View Details
154229-18-2 -
 73590-58-6 Omeprazole 98%View Details
73590-58-6 Omeprazole 98%View Details
73590-58-6 -
 201530-41-8 Deferasirox 98%View Details
201530-41-8 Deferasirox 98%View Details
201530-41-8 -
 Sertraline HCl 98%View Details
Sertraline HCl 98%View Details
79559-97-0
