Levonorgestrel
Synonym(s):Levonorgestrel;D (?)-Norgestrel;13β-Ethyl-17α-ethynyl-17β-hydroxygon-4-en-3-one;17α-Ethynyl-18-homo-19-nor-testosterone;18,19-Dinor-13β-ethyl-17β-hydroxy-4-pregnen-20-yn-3-one
- CAS NO.:797-63-7
- Empirical Formula: C21H28O2
- Molecular Weight: 312.45
- MDL number: MFCD06198806
- EINECS: 212-349-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
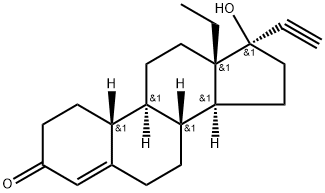
What is Levonorgestrel?
Absorption
Orally administered levonorgestrel is absorbed in the gastrointestinal tract while levonorgestrel administered through an IUD device is absorbed in the endometrium. Levonorgestrel is absorbed immediately in the interstitial fluids when it is inserted as a subdermal implant. After insertion of the subdermal implant, the Cmax of levonorgestrel is attained within 2-3 days.The Cmax following one dose of 0.75 mg of oral levonorgestrel is reached within the hour after administration, according to one reference. In a pharmacokinetic study of 1.5 mg of levonorgestrel in women with a normal BMI and those considered to be obese (BMI>30), mean Cmax was found to be 16.2 ng/mL and 10.5 ng/mL respectively. Tmax was found to be 2 hours for those with normal BMI and 2.5 hours for patients with increased BMI. The bioavailability of levonorgestrel approaches 100%.
Mean AUC has been shown to be higher in patients with a normal BMI, measuring at 360.1 h × ng/mL versus a range of 197.28 to 208.1 h × ng/mL in an obese group of patients. Obesity may contribute to decreased efficacy of levonorgestrel in contraception.
Toxicity
The oral LD50 in rats is greater than 5000 mg/kg.
An overdose of this drug, like other contraceptives, may cause nausea and withdrawal bleeding. Provide symptomatic treatment in the case of a levonorgestrel overdose and contact the local poison control center. There is no specific antidote for a levonorgestrel overdose.
Description
Levonorgestrel exhibits some androgenic activity but no glucocortic oid or antimineraloc orticoid action. Levonorgestrel can be administered orally, transdermally (combined with estradiol and formulated as a 7-day patch), and for prolonged, continuous use, via an intrauterine device (IUD). The oral bioavailability of levonorgestrel is approximately 95%. From a protein binding pers pective, 48% of an oral dose is bound to SHBG, and 50% is bound to albumin. Levonorgestrel under goes metabolic reduction of its ketone and is hydroxylated.
Chemical properties
White Solid
Originator
Ovrette,Wyeth,US,1968
The Uses of Levonorgestrel
An emergency contraceptive. Levonorgestrel is safe, tolerated and effective in emergency contraception in woman
The Uses of Levonorgestrel
progestin
The Uses of Levonorgestrel
D-(-)-Norgestrel is an emergency contraceptive. Levonorgestrel is safe, tolerated and effective in emergency contraception in woman. Norgestimate USP Related Compound A.
Background
Levonorgestrel (LNG) is a synthetic progestogen similar to Progesterone used in contraception and hormone therapy. Also known as Plan B, it is used as a single agent in emergency contraception, and as a hormonal contraceptive released from an intrauterine device, commonly referred to as an IUD. Some of these devices are known as Jaydess, Kyleena, and Mirena. A subdermal implant of levonorgestrel that slowly releases the hormone over a long-term period is also available. In addition to the above uses, levonorgestrel is used as a component of long-term combination contraceptives.
Globally, levonorgestrel is the most commonly used emergency contraceptive. It was initially granted FDA approval in 1982 and was the first emergency contraceptive containing only progesterone, showing high levels of efficacy and a lack of estrogenic adverse effects when compared to older emergency contraceptive regimens.
Indications
Emergency contraception
Levonorgestrel, in the single-agent emergency contraceptive form, is indicated for the prevention of pregnancy after the confirmed or suspected failure of contraception methods or following unprotected intercourse. It is distributed by prescription for patients under 17, and over the counter for those above this age. This levonorgestrel-only form of contraception is not indicated for regular contraception and must be taken as soon as possible within 72 hours after intercourse. It has shown a lower efficacy when it is used off label within 96 hours.
Long-term contraception or nonemergency contraception
In addition to the above indication in emergency contraception, levonorgestrel is combined with other contraceptives in contraceptive formulations designed for regular use, for example with ethinyl estradiol. It is used in various hormone-releasing intrauterine devices for long-term contraception ranging for a duration of 3-5 years. Product labeling for Mirena specifically mentions that it is recommended in women who have had at least 1 child and can be indicated for the prevention of pregnancy for up to 8 years. A subdermal implant is also available for the prevention of pregnancy for up to 5 years.
Hormone therapy and off-label uses
Levonorgestrel is prescribed in combination with estradiol as hormone therapy during menopause to manage vasomotor symptoms and to prevent osteoporosis.Off-label, levonorgestrel may be used to treat menorrhagia, endometrial hyperplasia, and endometriosis.
What are the applications of Application
Levonorgestrel is a synthetic progestin that binds to progesterone and androgen receptors
Definition
A synthetic steroid hormone used as an ingredient of oral contraceptives. Approved by FDA.
Manufacturing Process
To 0.7 gram of (+/-)-1,4-dihydro-17α-ethynyl-18-homo-oestradiol 3-methyl ether in 36 cc methanol was added 1.6 cc water and 2.4 cc concentrated hydrochloric acid. After standing at room temperature for 2 hours ether was added, and the washed and dried ethereal solution was evaporated, yielding a gum which was dissolved in 5 cc benzene and the solution absorbed on 50 grams of an activated fuller's earth. Elution with light petroleum containing increasing proportions of benzene gave a crystalline by-product: further elution with benzene containing a small proportion of ether gave a crystalline product which was recrystallized from ethyl acetate, yielding 0.11 gram of (+/-)-17α-ethynyl-18-homo-19-nortestosterone. MP 203° to 206°C.
brand name
Mirena (Berlex); Norplant (Wyeth); Plan B (Duramed).
Therapeutic Function
Progestin
Pharmacokinetics
Levonorgestrel prevents pregnancy by interfering with ovulation, fertilization, and implantation. The levonorgestrel-only containing emergency contraceptive tablet is 89% effective if it is used according to prescribing information within 72 hours after intercourse. The intrauterine and implantable devices releasing levonorgestrel are more than 99% in preventing pregnancy. Levonorgestrel utilized as a component of hormonal therapy helps to prevent endometrial carcinoma associated with unopposed estrogen administration.
Safety Profile
Human reproductive effects byingestion: menstrual cycle changes, fertility index.Questionable carcinogen with experimental neoplastigenicdata. When heated to decomposition it emits acrid smokeand irritating fumes.
Metabolism
After absorption of the oral emergency contraceptive preparation, levonorgestrel is conjugated and forms a large number of sulfate conjugates. In addition, glucuronide conjugates have been identified in the plasma. High levels of conjugated and unconjugated 3α, 5β-tetrahydrolevonorgestrel are found in the plasma. The entire metabolic pathway for levonorgestrel has not been studied, however, 16β-hydroxylation is one pathway that has been identified. Small quantities of 3α, 5α- tetrahydrolevonorgestrel and 16βhydroxylevonorgestrel are also formed. No active metabolites have been identified. The rate of metabolism may be considerably different according to the patient and may explain a wide variation in levonorgestrel clearance. Liver CYP3A4 and CYP3A5 hepatic enzymes are reported to be involved in the metabolism of levonorgestrel.
Properties of Levonorgestrel
| Melting point: | 206°C |
| Boiling point: | 392.36°C (rough estimate) |
| alpha | D20 -32.4° (c = 0.496 in CHCl3) |
| Density | 1.0697 (rough estimate) |
| refractive index | 1.4900 (estimate) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Practically insoluble in water, sparingly soluble in methylene chloride, slightly soluble in ethanol (96 per cent). |
| form | neat |
| pka | 13.09±0.40(Predicted) |
| form | Solid |
| color | Crystals from MeOH/CHCl3 |
| Water Solubility | 10mg/L(temperature not stated) |
| Merck | 13,6736 |
| CAS DataBase Reference | 797-63-7(CAS DataBase Reference) |
Safety information for Levonorgestrel
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity H362:Reproductive toxicity, effects on or via lactation |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Levonorgestrel
Levonorgestrel manufacturer
Kavya Pharma
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![Potassium (R)-[(3-ethoxy-1-methyl-3-oxoprop-1-enyl)amino]phenylacetate](https://img.chemicalbook.in/CAS/GIF/961-69-3.gif)
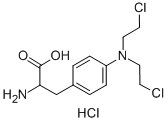

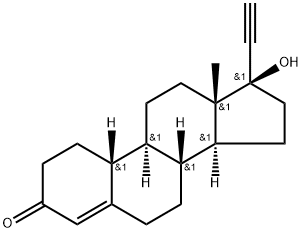
![(9R,10R,13S,17R)-13-ethyl-17-ethynyl-17-hydroxy-1,2,6,7,9,10,11,12,15,16-decahydrocyclopenta[a]phenanthren-3-one](https://img.chemicalbook.in/CAS/20180601/GIF/1260525-53-8.gif)
You may like
-
 Levonorgestrel 99%View Details
Levonorgestrel 99%View Details -
 Levonorgestrel 98%View Details
Levonorgestrel 98%View Details
797-63-7 -
 (-)-Norgestrel CAS 797-63-7View Details
(-)-Norgestrel CAS 797-63-7View Details
797-63-7 -
 Levonorgestrel APIView Details
Levonorgestrel APIView Details
797-63-7 -
 Levonorgestrel Api PowderView Details
Levonorgestrel Api PowderView Details
797-63-7 -
 Levonorgestrel CAS NO : 797-63-7View Details
Levonorgestrel CAS NO : 797-63-7View Details
797-63-7 -
 Levonorgestrel API PowderView Details
Levonorgestrel API PowderView Details
797-63-7 -
 LevonorgestrelView Details
LevonorgestrelView Details
797-63-7
