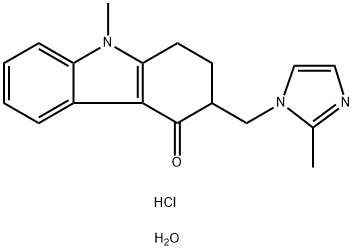
Ondansetron
Synonym(s):(RS)-1,2,3,9-Tetrahydro-9-methyl-3-(2-methylimidazol-1-ylmethyl)carbazol-4-one;1,2,3,9-Tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one hydrochloride;GR 38032F
- CAS NO.:99614-02-5
- Empirical Formula: C18H19N3O
- Molecular Weight: 293.36
- MDL number: MFCD00764297
- EINECS: 619-449-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 17:32:32

What is Ondansetron?
Absorption
Ondansetron is absorbed from the gastrointestinal tract and undergoes some limited first-pass metabolism . Mean bioavailability in healthy subjects, following administration of a single 8-mg tablet, was recorded as being approximately 56% to 60% . Bioavailability is also slightly enhanced by the presence of food .
Ondansetron systemic exposure does not increase proportionately to dose . The AUC from a 16-mg tablet was 24% greater than predicted from an 8-mg tablet dose . This may reflect some reduction of first-pass metabolism at higher oral doses .
Toxicity
At present, there is little information concerning overdosage with ondansetron . Nevertheless, there have been certain cases of somewhat idiosyncratic adverse effects associated with particular dosages of ondansetron used .
“Sudden blindness” (amaurosis) of 2 to 3 minutes duration plus severe constipation occurred in one patient that was administered 72 mg of ondansetron intravenously as a single dose . Hypotension (and faintness) occurred in another patient that took 48 mg of oral ondansetron . Following infusion of 32 mg over only a 4-minute period, a vasovagal episode with transient second-degree heart block was observed . Neuromuscular abnormalities, autonomic instability, somnolence, and a brief generalized tonic-clonic seizure (which resolved after a dose of benzodiazepine) were observed in a 12-month-old infant who ingested seven or eight 8-mg ondansetron tablets (approximately forty times the recommended 0.1-0.15 mg/kg dose for a pediatric patient) . In all instances, however, the events resolved completely .
The safety of ondansetron for use in human pregnancy has not been established . Ondansetron is not teratogenic in animals . However, as animal studies are not always predictive of human response, the use of ondansetron in pregnancy is not recommended .
Ondansetron is excreted in the milk of lactating rats . It is not known if it is excreted in human milk, however, nursing is not recommended during treatment with ondansetron .
Insufficient information is available to provide dosage recommendations for children 3 years of age or younger .
Description
Ondansetron (hydrochloride) (CRM) (Item No. 21710) is a certified reference material categorized as an antiemetic. Formulations containing ondansetron have been used to reduce alcohol consumption and mood disturbances in early-onset alcoholics. This product is intended for research and forensic applications.
Chemical properties
White to yellow crystal
Originator
Ondansetron hydrochloride ,Chemo Iberica , Spain
The Uses of Ondansetron
antibacterial
Indications
In the adult patient population:
i) orally administered ondansetron tablets and orally disintegrating tablets (ODT) are indicated for:
- the prevention of nausea and vomiting associated with emetogenic cancer chemotherapy, including high dose (ie. greater than or equal to 50 mg/m2) cisplatin therapy, and radiotherapy, and
- the prevention and treatment of postoperative nausea and vomiting
ii) intravenously administered ondansetron injection formulations are indicated for:
- the prevention of nausea and vomiting associated with emetogenic cancer chemotherapy, including high dose (ie. greater than or equal to 50 mg/m2) cisplatin therapy, and
- the prevention and treatment of postoperative nausea and vomiting
In the pediatric (4-18 years of age) patient population:
i) ondansetron was effective and well tolerated when given to children 4-12 years of age for the treatment of post-chemotherapy induced nausea and vomiting,
ii) ondansetron tablets, ondansetron ODT, ondansetron injection are not indicated for the treatment of children 3 years of age or younger,
iii) ondansetron tablets, ondansetron ODT, ondansetron injection are not indicated for use in any age group of the pediatric population for the treatment of post-radiotherapy induced nausea and vomiting, and
iV) ondansetron tablets, ondansetron ODT, ondansetron injection are not indicated for use in any age group of the pediatric population for the treatment of postoperative nausea and vomiting
In the geriatric (>65 years of age) patient population:
i) efficacy and tolerance of ondansetron were similar to that observed in younger adults for the treatment of post-chemotherapy and radiotherapy-induced nausea and vomiting, and
ii) clinical experience in the use of ondansetron in the prevention and treatment of postoperative nausea and vomiting is limited and is not indicated for use in the geriatric patient population
Background
A competitive serotonin type 3 receptor antagonist. It is effective in the treatment of nausea and vomiting caused by cytotoxic chemotherapy drugs, including cisplatin, and has reported anxiolytic and neuroleptic properties.
Having been developed in the 1980s by GlaxoSmithKline and approved by the US FDA since January 1991, ondansetron has demonstrated a long history of use and efficacy. Commonly formulated as oral tablets, orally disintegrating tablets (ODT), and injections, and available as generic products as well, ondansetron continues to see contemporary innovations in its formulation and use, including the development of orally soluble films that are both discreet in administration and less of a burden in comparison to having patients attempt to swallow pills during emesis.
The FDA withdrew its approval for the use of all intravenous drug products containing more than 16 mg of ondansetron hydrochloride in a single dose, due to a high risk of QT prolongation.
Indications
Ondansetron (Zofran) is potent antagonists of 5-HT3 receptors,which is found peripherally on vagal nerve terminals and centrally in the CTZ. During chemotherapy that induces vomiting, mucosal enterochromaffin cells in the GI tract release serotonin, which stimulates 5-HT3 receptors.
Definition
ChEBI: Ondansetron is a member of carbazoles.
Manufacturing Process
Preparation of 3-ethoxalyl-9-methyl-1,2,3,9-tetrahydro-4H-carbozol-4-one
3.0 g (0.13 mole) of sodium metal are portionwise added to a stirred mixture containing 19.93 g (0.1 mole) of 9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4one, 19.0 g (0.13 mole) of diethyl oxalate, 2 g of ethanol and 200 ml of dioxane. The slightly warming reaction mixture is stirred at 40° to 50°C for 4 hours, then 16 g of glacial acetic acid and finally 200 ml of water are added thereto at room temperature. After filtering off the yellow crystalline suspension, the precipitate is washed with water and dried to give the title compound in a yield of 24 g (80.2%), m.p. 118°-120°C
Preparation of 3-hydroxymethyl-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4one-3-glyoxylic acid lacton
After adding 0.1 g of triethylamine to a stirred suspension containing 3.00 g (0.01 mole) of the 3-ethoxalyl-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4
2512 one, in 20 ml of acetone, 1.13 g (0.015 mole) of formol solution are dropwise added to the mixture. The suspension becomes clear within 1 to 2 minutes and crystals begin to precipitate. After further stirring at 35° to 40°C for one hour, the reaction mixture is cooled down to room temperature, filtered off, the precipitate is washed with 50% acetone and dried to give 2.10 g (74.2%) of the title compound, m.p. 242°-244°C.
Preparation of ondansetron base (chemically 9-methyl-3-[(2-methyl-1-Himidazol-1-yl)methyl]-1,2,3,9-tetrahidro-4-H-carbazol-4-one)
A mixture containing 2.83 g (0.01 mole) of 3-hydroxymethyl-9-methyl-2,3,9tetrahydro-4H-carbazol-4-one-3-glyoxylic acid lactone, 15 ml of dioxane, 1.32 g of triethylamine, 1.0 g of ethanol and 1.64 g (0.02 mole) of 2methylimidazole is boiled under reflux while stirring for 5 hours. Thereafter, the reaction mixture is diluted with 45 ml of water and cooled down. The precipitate is filtered off, washed with aqueous dioxane and dried to obtain 2.56 g (87.3%) of the title compound, m.p. 220°-223°C.
Preparation of 9-methyl-3-[(2-methyl-1-H-imidazol-1-yl)methyl]-1,2,3,9tetrahydro-4H-carbazol-4-one hydrochloride dihydrate The process above described is followed, except that after cooling down the reaction mixture to room temperature after boiling, 20 ml of 37% aqueous hydrochloric acid are added thereto. Then, the precipitate is filtered off, washed with isopropanol and dried to obtain 2.40 g (65.6%) of the title salt, m.p. 178°-180°C. The active agent content of the product was found to be 100.3% based on potentiometric titration with sodium hydroxide solution. The theoretical water content is 9.85% (calculated for C18H19N3OHCl2H2O).The water content measured is 10.03%.
brand name
Zofran (GlaxoSmithKline).
Therapeutic Function
Serotonin antagonist
Pharmacokinetics
Ondansetron is a highly specific and selective serotonin 5-HT3 receptor antagonist, not shown to have activity at other known serotonin receptors and with low affinity for dopamine receptors , . The serotonin 5-HT3 receptors are located on the nerve terminals of the vagus in the periphery, and centrally in the chemoreceptor trigger zone of the area postrema , . The temporal relationship between the emetogenic action of emetogenic drugs and the release of serotonin, as well as the efficacy of antiemetic agents, suggest that chemotherapeutic agents release serotonin from the enterochromaffin cells of the small intestine by causing degenerative changes in the GI tract , . The serotonin then stimulates the vagal and splanchnic nerve receptors that project to the medullary vomiting center, as well as the 5-HT3 receptors in the area postrema, thus initiating the vomiting reflex, causing nausea and vomiting , .
Moreover, the effect of ondansetron on the QTc interval was evaluated in a double-blind, randomized, placebo and positive (moxifloxacin) controlled, crossover study in 58 healthy adult men and women . Ondansetron was tested at single doses of 8 mg and 32 mg infused intravenously over 15 minutes . At the highest tested dose of 32 mg, prolongation of the Fridericia-corrected QTc interval (QT/RR0.33=QTcF) was observed from 15 min to 4 h after the start of the 15 min infusion, with a maximum mean (upper limit of 90% CI) difference in QTcF from placebo after baseline-correction of 19.6 (21.5) msec at 20 min . At the lower tested dose of 8 mg, QTc prolongation was observed from 15 min to 1 h after the start of the 15-minute infusion, with a maximum mean (upper limit of 90% CI) difference in QTcF from placebo after baseline-correction of 5.8 (7.8) msec at 15 min . The magnitude of QTc prolongation with ondansetron is expected to be greater if the infusion rate is faster than 15 minutes . The 32 mg intravenous dose of ondansetron must not be administered . No treatment-related effects on the QRS duration or the PR interval were observed at either the 8 or 32 mg dose .
An ECG assessment study has not been performed for orally administered ondansetron . On the basis of pharmacokinetic-pharmacodynamic modelling, an 8 mg oral dose of ondansetron is predicted to cause a mean QTcF increase of 0.7 ms (90% CI -2.1, 3.3) at steady-state, assuming a mean maximal plasma concentration of 24.7 ng/mL (95% CI 21.1, 29.0) . The magnitude of QTc prolongation at the recommended 5 mg/m2 dose in pediatrics has not been studied, but pharmacokinetic-pharmacodynamic modeling predicts a mean increase of 6.6 ms (90% CI 2.8, 10.7) at maximal plasma concentrations .
In healthy subjects, single intravenous doses of 0.15 mg/kg of ondansetron had no effect on esophageal motility, gastric motility, lower esophageal sphincter pressure, or small intestinal transit time . Multiday administration of ondansetron has been shown to slow colonic transit in healthy subjects . Ondansetron has no effect on plasma prolactin concentrations .
Clinical Use
Ondansetron
Side Effects
This causes vagal afferent discharge, inducing vomiting. In binding to 5-HT3 receptors, ondansetron blocks serotonin stimulation, hence vomiting, after emetogenic stimuli such as cisplatin. Headache is the most frequently reported adverse effect of these medications.
Safety Profile
A poison by intravenous route.Human systemic effects by intravenous route: jaundice.When heated to decomposition it emits toxic vapors ofNOx.
Veterinary Drugs and Treatments
Used as an antiemetic when conventional antiemetics are ineffective, such as when administering cisplatin or for other causes of intractable vomiting. The use of ondansetron in cats is somewhat controversial and some state it should not be used in this species.
Drug interactions
Potentially hazardous interactions with other drugs
Cytotoxics: possible increased risk of ventricular
arrhythmias with panobinostat and vandetanib.
Dopaminergics: possible increased risk of
hypotension with apomorphine - avoid.
Metabolism
Ondansetron is metabolised in the liver through multiple enzymatic pathways; it is a substrate for cytochrome P450 isoenzymes, primarily CYP3A4, but also CYP1A2 and CYP2D6. The metabolites do not contribute to the pharmacological activity of ondansetron. Less than 5% of a dose is excreted unchanged in the urine
Properties of Ondansetron
| Melting point: | 231-232° |
| Boiling point: | 435.21°C (rough estimate) |
| Density | 1.1385 (rough estimate) |
| refractive index | 1.5855 (estimate) |
| storage temp. | −20°C |
| solubility | H2O: >5 mg/mL |
| form | solid |
| pka | pKa 7.4 (Uncertain) |
| color | white |
| CAS DataBase Reference | 99614-02-5(CAS DataBase Reference) |
Safety information for Ondansetron
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H318:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment |
Computed Descriptors for Ondansetron
Ondansetron manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 ONDANSETRON HCL 99%View Details
ONDANSETRON HCL 99%View Details -
 Ondansetron 99614-02-5 98%View Details
Ondansetron 99614-02-5 98%View Details
99614-02-5 -
 Ondansetron 99614-02-5 99%View Details
Ondansetron 99614-02-5 99%View Details
99614-02-5 -
 Ondansetron 98%View Details
Ondansetron 98%View Details
99614-02-5 -
 Ondansetron 98% CAS 99614-02-5View Details
Ondansetron 98% CAS 99614-02-5View Details
99614-02-5 -
 Ondansetron impurity standard CAS 99614-02-5View Details
Ondansetron impurity standard CAS 99614-02-5View Details
99614-02-5 -
 Ondansetron CAS 99614-02-5View Details
Ondansetron CAS 99614-02-5View Details
99614-02-5 -
 Ondansetron Hcl Di HydrateView Details
Ondansetron Hcl Di HydrateView Details
99614-02-5
