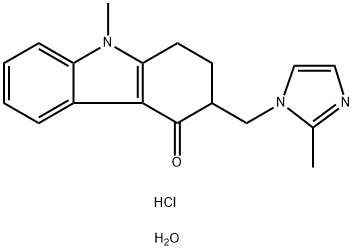
Ondansetron hydrochloride
Synonym(s):1,2,3,9-Tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one hydrochloride;GR 38032F;Ondansetron hydrochloride dihydrate
- CAS NO.:103639-04-9
- Empirical Formula: C18H22ClN3O2
- Molecular Weight: 347.84
- MDL number: MFCD00833882
- EINECS: 600-465-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 21:30:20
What is Ondansetron hydrochloride?
Description
Ondansetron hydrochloride is a selective 5HT3-antagonist approved for the management of nausea and vomiting induced by cancer chemotherapy and radiotherapy. It is reported to have a superior side-effect profile compared with metoclopramide, representing an important advance in the treatment of nausea and vomiting associated with chemotherapy. Ondansetron hydrochloride is the first specific 5HT3-antagonist to reach the market. It is also under investigation for a number of other indications, including anxiety and schizophrenia.
Chemical properties
White or almost white powder.
Originator
Glaxo (United Kingdom)
The Uses of Ondansetron hydrochloride
Ondansetron hydrochloride dihydrate has been used as a 5-HT3 antagonist to attenuate rostro ventromedial medulla (RVM)/cholecystokinin (CCK)-induced mechanical hypersensitivity and to block the effects of trichostatin A (TSA) on 5-HT3 protein.
The Uses of Ondansetron hydrochloride
Antiemetic;Serotonergic receptor antagonist
The Uses of Ondansetron hydrochloride
A serotonin type 3 receptor antagonist.
The Uses of Ondansetron hydrochloride
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
What are the applications of Application
Ondansetron hydrochloride is a selective SR-3 antagonist
Definition
ChEBI: Ondansetron hydrochloride is a member of carbazoles.
Manufacturing Process
Preparation of 3-ethoxalyl-9-methyl-1,2,3,9-tetrahydro-4H-carbozol-4-one
3.0 g (0.13 mole) of sodium metal are portionwise added to a stirred mixture containing 19.93 g (0.1 mole) of 9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4one, 19.0 g (0.13 mole) of diethyl oxalate, 2 g of ethanol and 200 ml of dioxane. The slightly warming reaction mixture is stirred at 40° to 50°C for 4 hours, then 16 g of glacial acetic acid and finally 200 ml of water are added thereto at room temperature. After filtering off the yellow crystalline suspension, the precipitate is washed with water and dried to give the title compound in a yield of 24 g (80.2%), m.p. 118°-120°C
Preparation of 3-hydroxymethyl-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4one-3-glyoxylic acid lacton
After adding 0.1 g of triethylamine to a stirred suspension containing 3.00 g (0.01 mole) of the 3-ethoxalyl-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4
2512 one, in 20 ml of acetone, 1.13 g (0.015 mole) of formol solution are dropwise added to the mixture. The suspension becomes clear within 1 to 2 minutes and crystals begin to precipitate. After further stirring at 35° to 40°C for one hour, the reaction mixture is cooled down to room temperature, filtered off, the precipitate is washed with 50% acetone and dried to give 2.10 g (74.2%) of the title compound, m.p. 242°-244°C.
Preparation of ondansetron base (chemically 9-methyl-3-[(2-methyl-1-Himidazol-1-yl)methyl]-1,2,3,9-tetrahidro-4-H-carbazol-4-one)
A mixture containing 2.83 g (0.01 mole) of 3-hydroxymethyl-9-methyl-2,3,9tetrahydro-4H-carbazol-4-one-3-glyoxylic acid lactone, 15 ml of dioxane, 1.32 g of triethylamine, 1.0 g of ethanol and 1.64 g (0.02 mole) of 2methylimidazole is boiled under reflux while stirring for 5 hours. Thereafter, the reaction mixture is diluted with 45 ml of water and cooled down. The precipitate is filtered off, washed with aqueous dioxane and dried to obtain 2.56 g (87.3%) of the title compound, m.p. 220°-223°C.
Preparation of 9-methyl-3-[(2-methyl-1-H-imidazol-1-yl)methyl]-1,2,3,9tetrahydro-4H-carbazol-4-one hydrochloride dihydrate The process above described is followed, except that after cooling down the reaction mixture to room temperature after boiling, 20 ml of 37% aqueous hydrochloric acid are added thereto. Then, the precipitate is filtered off, washed with isopropanol and dried to obtain 2.40 g (65.6%) of the title salt, m.p. 178°-180°C. The active agent content of the product was found to be 100.3% based on potentiometric titration with sodium hydroxide solution. The theoretical water content is 9.85% (calculated for C18H19N3OHCl2H2O).The water content measured is 10.03%.
brand name
Zofran (GlaxoSmithKline);Zophran.
Therapeutic Function
Serotonin antagonist
General Description
Ondansetron Hydrochloride acts as a 5HT3 (serotonin) antagonist and is a powerful antiemetic drug. It is widely used for the prevention and treatment of nausea and vomiting related to post-operative states, cancer chemotherapy and radiotherapy, and chronic medical illness. It has been found to exhibit inconsistent bioavailability resulting from its poor aqueous solubility and high first-pass hepatic metabolism.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Biological Activity
Selective 5-HT 3 receptor antagonist (K i = 6.16 nM). Antiemetic; prevents emesis induced by cytotoxic drugs and radiation.
Biochem/physiol Actions
5-HT3 serotonin receptor antagonist
Storage
Room temperature
Properties of Ondansetron hydrochloride
| Melting point: | 231-232 C |
| storage temp. | -20°C |
| solubility | H2O: >5mg/mL |
| form | powder |
| color | white |
| CAS DataBase Reference | 103639-04-9(CAS DataBase Reference) |
Safety information for Ondansetron hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H318:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Ondansetron hydrochloride
| InChIKey | FELGMEQIXOGIFQ-ZDUSSCGKSA-N |
Ondansetron hydrochloride manufacturer
Global Chemie
Bazayan & Co.
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


![3-[(Dimethylamino)methyl]-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one](https://img.chemicalbook.in/CAS/GIF/153139-56-1.gif)




You may like
-
 103639-04-9 98%View Details
103639-04-9 98%View Details
103639-04-9 -
 Ondansetron Hydrochloride 98%View Details
Ondansetron Hydrochloride 98%View Details
103639-04-9 -
 Ondansetron hydrochloride dihydrate 98%View Details
Ondansetron hydrochloride dihydrate 98%View Details
103639-04-9 -
 Ondansetron Hyd 99%View Details
Ondansetron Hyd 99%View Details -
 Ondansetron Hydrochloride Dihydrate CAS 103639-04-9View Details
Ondansetron Hydrochloride Dihydrate CAS 103639-04-9View Details
103639-04-9 -
 Ondansetron hydrochloride dihydrate 98% (HPLC) CAS 103639-04-9View Details
Ondansetron hydrochloride dihydrate 98% (HPLC) CAS 103639-04-9View Details
103639-04-9 -
 ONDANSETRON HYDROCHLORIDE CAS 103639 04 9, 4 mgView Details
ONDANSETRON HYDROCHLORIDE CAS 103639 04 9, 4 mgView Details
103639-04-9 -
 CAS 103639 04 9 ONDANSETRON HYDROCHLORIDE, 4 mgView Details
CAS 103639 04 9 ONDANSETRON HYDROCHLORIDE, 4 mgView Details
103639-04-9
