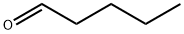Octanal
Synonym(s):Aldehyde C8;Caprylaldehyde, Octaldehyde;Caprylic aldehyde;Octanal;Octyl aldehyde
- CAS NO.:124-13-0
- Empirical Formula: C8H16O
- Molecular Weight: 128.21
- MDL number: MFCD00007029
- EINECS: 204-683-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is Octanal?
Description
May be prepared by oxidation of the corresponding alcohol or reduction of the corresponding acid; also from coconut fatty acids via methyl-n-octanoate.
Chemical properties
Octanal occurs in several citrus oils, for example, orange oil. It is a colorless liquid with a pungent odor, which becomes citrus-like on dilution. Octanal is used in perfumery in low concentrations, in eau de cologne, and in artificial citrus oils.
Chemical properties
n-Octanal has a fatty, citrus, honey odor on dilution.
Occurrence
Reported found in the essential oils of sweet orange, bitter orange, mandarin, tangerine, grapefruit, Mexican lime, lemon, Taiwan citronella, rose, lemongrass, Pinus sabiniana, Pinus jefferyi, Xanthoxylum rhetsa, lime petitgrain, clary sage, lavandin, and others. Also reported in over 180 foods and beverages including apple, apricot, many berries, guava, grapes, melon, papaya, celery, peas, potato, potato chips, tomato, ginger, spearmint oil, cheeses, butter, milk, cooked egg, fish and fish oil, meats, hop oil, beer, rum, cider, white wine, cocoa, tea, roasted filberts and peanuts, pecans, oats, coconut products, soybean, avocado, passion fruit, starfruit, beans, mushroom, trassi, macadamia nut, sesame seed, mango, cauliflower, tamarind, fig, calamus, rice, sweet potato, dill, lovage, caraway seed, corn oil, corn tortillas, loquat, shrimp, lobster, oyster, crab, crayfish, clam, maté, angelica root oil and mastic gum oil.
The Uses of Octanal
Octanal is an aromatic aldehyde often found in citrus oils. It is used commercially as a component in perfumes and in flavor production for the food industry.
What are the applications of Application
Octanal is an aromatic aldehyde often found in citrus oils
Definition
ChEBI: A fatty aldehyde formally arising from reduction of the carboxy group of caprylic acid (octanoic acid).
Preparation
By oxidation of the corresponding alcohol or reduction of the corresponding acid; also from coconut fatty acids via methyl-n-octoate.
Aroma threshold values
Detection: 1.4 to 6.4 ppb
Taste threshold values
Taste characteristics at 25 ppm: aldehyde, green with a peely, citrus, orange note.
Synthesis Reference(s)
Journal of the American Chemical Society, 99, p. 3837, 1977 DOI: 10.1021/ja00453a053
Journal of Heterocyclic Chemistry, 29, p. 257, 1992 DOI: 10.1002/jhet.5570290148
Tetrahedron Letters, 36, p. 2247, 1995 DOI: 10.1016/0040-4039(95)00262-B
General Description
Colorless liquids with a strong fruity odor. Less dense than water and insoluble in water. Flash points 125°F. Used in making perfumes and flavorings.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
OCTYL ALDEHYDES are aldehydes. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation.
Health Hazard
Inhalation may be irritating to mucous membrane; overexposure may cause dizziness and collapse. Ingestion causes irritation of mouth and stomach. Contact with eyes or skin causes irritation.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Flammable
Safety Profile
Mildly toxic by ingestion and skin contact. A skin and eye irritant. Flammable liquid when exposed to heat, sparks, or flame. Can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical. See also ALDEHYDES.
Metabolism
Aldehydes C-8, C-10, C-12 and C-14 (myristic), the lower unsubstituted aliphatic aldehydes, are readily oxidized in the animal body to the corresponding fatty acids, which normally undergo oxidation and are eventially oxidized to carbon dioxide and water.
Properties of Octanal
| Melting point: | 12-15 °C(lit.) |
| Boiling point: | 171 °C(lit.) |
| Density | 0.822 g/mL at 20 °C |
| vapor pressure | 2 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 2797 | OCTANAL |
| Flash point: | 125 °F |
| storage temp. | Store below +30°C. |
| solubility | 0.21g/l |
| form | Liquid |
| color | Clear colorless to pale yellow |
| Odor | fatty-orange odor |
| explosive limit | 1.0-6.5%(V) |
| Odor Threshold | 0.00001ppm |
| Water Solubility | slightly soluble |
| Sensitive | Air Sensitive |
| Merck | 14,1766 |
| JECFA Number | 98 |
| BRN | 1744086 |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents, strong reducing agents, strong bases. |
| CAS DataBase Reference | 124-13-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Octanal(124-13-0) |
| EPA Substance Registry System | Octanal (124-13-0) |
Safety information for Octanal
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Octanal
| InChIKey | NUJGJRNETVAIRJ-UHFFFAOYSA-N |
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 n-Octanal CAS 124-13-0View Details
n-Octanal CAS 124-13-0View Details
124-13-0 -
 Octanal 98% CAS 124-13-0View Details
Octanal 98% CAS 124-13-0View Details
124-13-0 -
 Octanal CAS 124-13-0View Details
Octanal CAS 124-13-0View Details
124-13-0 -
 Octanal CAS 124-13-0View Details
Octanal CAS 124-13-0View Details
124-13-0 -
 Octanal CAS 124-13-0View Details
Octanal CAS 124-13-0View Details
124-13-0 -
 124-13-0 OCTYLDEHYDE 98%View Details
124-13-0 OCTYLDEHYDE 98%View Details
124-13-0 -
 124-13-0 99%View Details
124-13-0 99%View Details
124-13-0 -
 609-15-4View Details
609-15-4View Details
609-15-4