1-Octanol
Synonym(s):1-Octanol;Octyl alcohol;Capryl alcohol;n-Octyl alcohol;n-Octyl Alcohol, N-Octanol
- CAS NO.:111-87-5
- Empirical Formula: C8H18O
- Molecular Weight: 130.23
- MDL number: MFCD00002988
- EINECS: 203-917-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
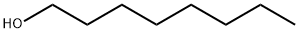
What is 1-Octanol?
Description
1-Octanol has a fresh, orange-rose odor that is quite sweet with an oily, sweet, slightly herbaceous taste. It may be prepared by reduction of some caprylic esters such as methyl caprylate with sodium ethoxide.
Description
1-Octanol (111-87-5) is a colorless and transparent liquid with the chemical formula C8H18O. It is a fatty alcohol consisting of a linear, saturated chain of eight carbon atoms. Although 1-Octanol is not highly soluble in water, it can dissolve easily in alcohol, ether, and chloroform. This compound is commonly used as a raw material in the production of fragrances, octanal, octanoic acid, and its esters. It also functions as a solvent, defoamer, and lubricating oil additive. Notably, 1-Octanol poses a low level of toxicity, but it can be irritating to the skin and eyes. Nonetheless, it is considered safe to use under normal conditions because of its low vapor pressure.
Chemical properties
1-Octanol has a fresh, orange-rose odor, quite sweet and an oily, sweet, slightly herbaceous taste.
Occurrence
Reported as frequently occurring in essential oils as an ester; the free alcohol has been reported found in the essential oils of green tea, grapefruit, California orange, Andropogon intermedius, Heracleum villosum, violet leaves, Anethum graveolens and bitter orange. Also reported in over 200 foods and beverages including apple, apricot, banana, citrus peel oils and juices, many berries, guava, grapes, melon, papaya, peach, pear, asparagus, kohlrabi, leek, peas, tomato, potato, clove, ginger, mustard, spearmint oil, wheat bread, many cheeses, cooked egg, butter, milk, fish, meats, beer, rum, whiskies, cognac, cider, sherry, grape wines, cocoa, tea, nuts, honey, soybean, coconut, passion fruit, olive, avocado, plum, rose apple, beans, mushroom, starfruit, Bantu beer, sesame seed, cauliflower, broccoli, tamarind, fruit brandies, fig, cardamom, coriander seed and leaf, rice, quince, litchi, dill, lovage, sweet corn, corn oil, malt, kiwifruit, loquat, Bourbon vanilla, clary sage, oysters, crayfish, clam and Chinese quince.
The Uses of 1-Octanol
1-Octanol is an fatty acid alcohol that is used industrially to form various synthetic intermediate and pharmaceuticals.
The Uses of 1-Octanol
1-Octanol is used as a precursor to prepare esters, which is used in perfumes and flavorings. It is used as food additive, anti-foaming agent and emulsifier in anti-rust emulsions. It is widely involved in chemical industry for the synthesis of ethoxylates, alkyl sulfates and ether sulfates. It is used as a solvent in paints, varnishes, waxes, and surface coatings and mainly involved in agricultural chemistry to inhibit excessive growth of tobacco plants.
What are the applications of Application
1-Octanol is a T-type calcium channel blocker
Definition
ChEBI: Octan-1-ol is an octanol carrying the hydroxy group at position 1. It has a role as a plant metabolite. It is an octanol and a primary alcohol.
Preparation
1-Octanol may be prepared by reduction of some caprylic esters such as methyl caprylate with sodium ethoxide.
Production Methods
1-Octanol is made commercially by sodium reduction or high-pressure catalytic hydrogenation of the esters of naturally occurring caprylic acid or by oligomerization of ethylene using aluminum alkyl technology.
What are the applications of Application
1-octanol is mainly used in the production of plasticizer, extractant and stabilizer, and as an intermediate of solvent and perfume. In the field of plasticizers, 1-octanol generally refers to 2-ethylhexanol, which is a bulk raw material of one million tons and is far more valuable than n-octanol in industry. Octanol itself is also used as a spice, blending roses, lilies and other flower fragrance as a soap flavor. This product is an edible spice that is allowed to be used according to Chinese GB2760-86. Mainly used to make coconut, pineapple, peach, chocolate and citrus flavors.
Aroma threshold values
Detection: 42 to 480 ppb
Taste threshold values
Taste characteristics at 2 ppm: waxy, green, citrus, orange and aldehydic with a fruity nuance.
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 38, p. 2097, 1990 DOI: 10.1248/cpb.38.2097
Tetrahedron Letters, 23, p. 157, 1982 DOI: 10.1016/S0040-4039(00)86773-8
General Description
A clear colorless liquid with a penetrating aromatic odor. Insoluble in water and floats on water. Vapors heavier than air. Vapors may irritate the eyes, nose, and respiratory system.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Attacks plastics [Handling Chemicals Safely 1980. p. 236]. Acetyl bromide reacts violently with alcohols or water [Merck 11th ed. 1989]. Mixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Example: an explosion will occur if dimethylbenzylcarbinol is added to 90% hydrogen peroxide then acidified with concentrated sulfuric acid. Mixtures of ethyl alcohol with concentrated hydrogen peroxide form powerful explosives. Mixtures of hydrogen peroxide and 1-phenyl-2-methyl propyl alcohol tend to explode if acidified with 70% sulfuric acid [Chem. Eng. News 45(43):73. 1967; J, Org. Chem. 28:1893. 1963]. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous-carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M. 1991]. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence [Wischmeyer 1969].
Health Hazard
Irritates skin and eyes.
Flammability and Explosibility
Not classified
Biochem/physiol Actions
Blocks T-type Ca2+ channels
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion. Mutation data reported. A skin irritant. Combustible liquid when exposed to heat or flame; can react with oxidizing materials. To fight fire, use water foam, fog, alcohol foam, dry chemical, CO2. See also ALCOHOLS.
Synthesis
1-octanol exists in essential oils such as bitter orange, grapefruit, sweet orange, green tea and violet leaves in free state, or in acetate, butyrate and isovalerate. In industrial production, octanal can be reduced or prepared by using octanoic acid in coconut oil. It can also be prepared by carbonyl synthesis with heptene-1 as raw material. HEPTENE, carbon monoxide and hydrogen form aldehyde in the presence of cobalt salt at 150-170 ℃ and high pressure of 20-30Mpa. After cobalt removal, HEPTENE is hydrogenated to primary alcohol under pressure with nickel catalyst. This method has mature production technology abroad.
Carcinogenicity
There was no evidence of tumors
in the cancer screening lung adenoma study in which mice
were injected intraperitoneally with 100 and 500 mg/kg 1-
octanol three times a week for 8 weeks. This assay has
not been validated as a reliable screen for cancer.
1-Octanol is a weak skin tumor promoter when applied
three times a week for 60 weeks to mice skin that had been
initiated with dimethylbenz[a]anthracene.
Metabolism
The primary aliphatic alcohols undergo two general reactions in vivo, namely oxidation to carboxylic acids and direct conjugation with glucuronic acid. The first reaction proceeds with the intermediate formation of an aldehyde, and the carboxylic acid from this may be either oxidized completely to carbon dioxide or excreted as such or combined with glucuronic acid as an ester glucuronide. The extent to which an alcohol undergoes the second reaction, i.e. direct conjugation to an ether glucuronide, appears to depend upon the speed of the first reaction, for alcohols which are rapidly oxidized form very little ether glucuronide unless given in high doses (Williams, 1959).
Purification Methods
Fractionally distil it under reduced pressure. Dry it with sodium and again fractionally distil or reflux with boric anhydride and re-distilled (b 195-205o/5mm), the distillate being neutralised with NaOH and again fractionally distilled. Also purify it by distillation from Raney nickel and by preparative GLC. [Beilstein 1 IV 1756.]
Properties of 1-Octanol
| Melting point: | −15 °C(lit.) |
| Boiling point: | 196 °C(lit.) |
| Density | 0.827 g/mL at 25 °C(lit.) |
| vapor density | 4.5 (vs air) |
| vapor pressure | 0.14 mm Hg ( 25 °C) |
| refractive index | n |
| FEMA | 2800 | 1-OCTANOL |
| Flash point: | 178 °F |
| storage temp. | Store below +30°C. |
| solubility | water: partially soluble107g/L at 23°C |
| form | liquid |
| pka | 15.27±0.10(Predicted) |
| color | APHA: ≤10 |
| Odor | Alcohol like |
| Relative polarity | 0.537 |
| explosive limit | 0.8%(V) |
| Odor Threshold | 0.0027ppm |
| Water Solubility | insoluble |
| λmax | λ: 215 nm Amax: 1.00 λ: 225 nm Amax: 0.50 λ: 235 nm Amax: 0.20 λ: 250 nm Amax: 0.05 λ: 300-400 nm Amax: 0.01 |
| Merck | 14,6751 |
| JECFA Number | 97 |
| BRN | 1697461 |
| Dielectric constant | 10.3(20℃) |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 111-87-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Octanol(111-87-5) |
| EPA Substance Registry System | 1-Octanol (111-87-5) |
Safety information for 1-Octanol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. P501:Dispose of contents/container to..… |
Computed Descriptors for 1-Octanol
| InChIKey | KBPLFHHGFOOTCA-UHFFFAOYSA-N |
1-Octanol manufacturer
CEFA CILINAS BIOTICS PVT LTD
Gayatri Industries
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 N OCTANOL 99%View Details
N OCTANOL 99%View Details -
 Octan-1-ol CAS 111-87-5View Details
Octan-1-ol CAS 111-87-5View Details
111-87-5 -
 Octanol CASView Details
Octanol CASView Details -
 Caprylic alcohol CAS 111-87-5View Details
Caprylic alcohol CAS 111-87-5View Details
111-87-5 -
 1-Octanol (1-Octyl Alcohol) pure CAS 111-87-5View Details
1-Octanol (1-Octyl Alcohol) pure CAS 111-87-5View Details
111-87-5 -
 1-Octanol (1-Octyl Alcohol) for HPLC & UV Spectroscopy CAS 111-87-5View Details
1-Octanol (1-Octyl Alcohol) for HPLC & UV Spectroscopy CAS 111-87-5View Details
111-87-5 -
 1-Octanol CAS 111-87-5View Details
1-Octanol CAS 111-87-5View Details
111-87-5 -
 1 Octanol chemical, For Pharmaceutical intermediates, 200 litre DrumView Details
1 Octanol chemical, For Pharmaceutical intermediates, 200 litre DrumView Details
111-87-5