o-Phthalaldehyde
Synonym(s):OPA;o-Phthalaldehyde;Phthaldialdehyde;o-Phthalic dicarboxaldehyde;o-Phthalaldehyde
- CAS NO.:643-79-8
- Empirical Formula: C8H6O2
- Molecular Weight: 134.13
- MDL number: MFCD00003335
- EINECS: 211-402-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-21 20:59:21
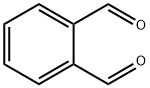
What is o-Phthalaldehyde?
Chemical properties
o-Phthalaldehyde is a pale yellow crystalline solid.

o-Phthalaldehyde is mainly used as a high-level disinfectant (a low-temperature chemical method) for heat-sensitive medical and dental equipment such as endoscopes and thermometers; in recent years, it has gained popularity as a safe and better alternative to glutaraldehyde.
There are some researches show, pH7.5 contains the sterilizing agent of o-phthalaldehyde 0.5%, and its sterilizing power, sterilization speed, stability and toxicity all are better than glutaraldehyde, can kill mycobacterium in the 5min, the bacterium number reduces by 5 logarithmic value, and o-phthalaldehyde is very stable, tasteless in pH3~9 scopes, non-stimulated to human nose, eye mucosa, and need not activate before using, various materials are had good consistency, have tangible microbiocidal activity.
The Uses of o-Phthalaldehyde
o-Phthalaldehyde can be widely used for precolumn derivatization of amino acids in HPLC separation or Capillary electrophoresis. For flow cytometric measurements of protein thiol groups.
The Uses of o-Phthalaldehyde
Precolumn derivatization reagent for primary amines and amino acids. The fluorescent derivative can be detected by reverse-phase HPLC. The reaction requires OPA, primary amine and a sulfhydryl. In the presence of excess sulfhydryl, amines can be quantitated. In the presence of excess amine, sulfhydryls can be quantitated.
The Uses of o-Phthalaldehyde
Disinfectant. Reagent in fluorometric determination of primary amines and thiols.
What are the applications of Application
2-Phthalaldehyde is a compound used in precolumn derivatization of amino acids for HPLC separation
Definition
ChEBI: A dialdehyde in which two formyl groups are attached to adjacent carbon centres on a benzene ring.
Preparation
o-Phthalaldehyde is a high-level chemical disinfectant that is commonly used for disinfection of dental and medical instruments as an alternative to glutaraldehyde, which is a known skin and respiratory sensitizer.
A variety of processes for manufacturing o-phthalaldehyde have been reported in the literature.
o-Phthalaldehyde is produced by heating pure benzaldehyde and chloroform with potassium hydroxide solution. The resulting solution is further acidified with hydrochloric acid and cooled to yield a colorless powder of o-phthalaldehyde.
It is also produced by ozonization of naphthalene in alcohol followed by catalytic hydrogenation.
Catalytic oxidation of various chemicals is also used in manufacturing o-phthalaldehyde. o-Phthalaldehyde can be manufactured by oxidation of phthalan by nitrogen monoxide in acetonitrile with N-hydroxyphthalimide as the catalyst to yield 80% to 90%.
Definition
Using o-phthalaldehyde (OPA) in combination with a thiol reagent is a standard method for detecting primary amines in amino acids, peptides, and proteins. O-phthalaldehyde, in the presence of 2-mercaptoethanol, reacts with primary amines to form highly fluorescent products. Picomole quantities of amino acids, peptides, and proteins can be detected easily. o-Phthalaldehyde is five to ten times more sensitive than fluorescamine and is soluble and stable in aqueous buffers. Despite its widespread use, the exact reaction mechanism has been debated since the 1980s. Rovelli's results support the mechanism originally proposed by Sternson and Wong, in which the primary amine first reacts with OPA, followed by a reaction with the thiol to form the fluorescent isoindole product[1-2].
Synthesis Reference(s)
Journal of the American Chemical Society, 73, p. 1668, 1951 DOI: 10.1021/ja01148a076
Tetrahedron Letters, 27, p. 1793, 1986 DOI: 10.1016/S0040-4039(00)84377-4
Reactivity Profile
o-Phthalaldehyde (OPA)-amine reaction and OPA-amine-thiol reaction have been developed to effectively modify native peptides and proteins under the physiological conditions. First, OPA and its derivatives can rapidly and smoothly react with primary amine moieties in peptides and proteins to achieve native protein biconjugations. Furthermore, OPA-alkyne bifunctional linkers can be used for proteome profiling. Second, OPA-amine-thiol three-component reaction has been developed for chemoselective peptide cyclization, directly on unprotected peptides in the aqueous buffer. Moreover, this OPA-guided cyclic peptide can be further modified with the N-maleimide moiety in one pot to introduce additional functionalities[3].
Flammability and Explosibility
Not classified
Biotechnological Applications
O-phthalaldehyde(OPA) is used for precolumn derivatization of amino acids for HPLC separation and for flow cytometric measurements of protein thiol groups. Used for fluorometric determination of histamine, histidine and other amino acids. Also used for cholesterol assay in the picomole range.
Phthaldialdehyde has been used:
in the preparation of O-phthaldialdehyde reagent for analysing gentamycin content.
in the preparation of reagent for determining the degree of hydrolysis of milk proteins.
in the measurement of free amino acids of milk samples by O-phthaldialdehyde/N-acetyl-L-cysteine (OPA/NAC) assay.
in the derivatization of putrescine samples.
Potential Exposure
The primary routes of human exposure to o-phthalaldehyde are by inhalation and through the skin, which may occur through accidental or occupational exposures. Along with its increasing popularity as a chemical sterilizer, o-phthalaldehyde has many applications in analytical methods and in diagnostic kits. o-Phthalaldehyde is also used as an intermediate in the synthesis of pharmaceuticals and as a reagent in the tanning industry, hair colorings, wood treatment, and antifouling paints. o-Phthalaldehyde was approved for use as an indoor antimicrobial pesticide in 1997; however, it is no longer registered with the United States Environmental Protection Agency (USEPA) for this use.
Chemical properties
o-Phthalaldehyde dissolves in water solution at pH < 11.5. Its solutions degrade upon UV illumination and exposure to air.
Carcinogenicity
No information on the carcinogenicity of o-phthalaldehyde in experimental animals or humans was found in a review of the literature.
Characteristics
O-phthalaldehyde(OPA) (OPA) is the new and effective chemosterilant of one abroad developed in recent years, there is wide spectrum, efficient, the advantage of low corrosion, also there is the characteristics such as zest is little, concentration is low, and the most isolated glutaraldehyde resistance mycobacteria is had good killing action.
Toxicity
Genetic Toxicity
Microbial Gene Mutation:99% Pure OPA: negative in S. typhimurium strains TA98, TA102, TA104, TA1535, and TA1537 with or without metabolic activation (WATCH subgroup, 2003)
Gene Mutation: 99-99.7% Pure OPA: negative for mutation at the thymidine kinase locus in mouse lymphoma cells and the HGPRT locus in CHO cells with and without metabolic activation; negative for unscheduled DNA synthesis in rat hepatocytes: 99-99.7% Pure OPA: induced chromosome aberrations and sister chromatid exchanges in CHO cells with and without metabolic activation; negative for chromosome aberrations in rat bone marrow cells (WATCH subgroup, 2003)
References
[1] Grazia Rovelli, Kevin R Wilson. “Elucidating the Mechanism for the Reaction of o-Phthalaldehyde with Primary Amines in the Presence of Thiols.” The Journal of Physical Chemistry B 127 14 (2023): 3257–3265.
[2] J R Benson, ;P E Hare. “O-phthalaldehyde: fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin.” Proceedings of the National Academy of Sciences of the United States of America 72 2 (1975): 619–22.
[3] “Chemical Tools for Imaging, Manipulating, and Tracking Biological Systems: Diverse Methods for Optical Imaging and Conjugation.” Methods in enzymology 1 1 (1900).
Properties of o-Phthalaldehyde
| Melting point: | 55-58 °C(lit.) |
| Boiling point: | 83-84 °C (0.7501 mmHg) |
| Density | 1.13 |
| vapor pressure | 0.56Pa at 25℃ |
| refractive index | 1.4500 (estimate) |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | The solubility of o-phthalaldehyde is 3g/100 mL diisopropyl ether, 5g/100mL deionized water, 20g/100mL chloroform, or 20g/100mL acetone at 20°C. |
| form | powder |
| color | yellow |
| PH | 7 (53g/l, H2O, 20℃) |
| Water Solubility | soluble |
| Sensitive | Air Sensitive |
| Merck | 14,7368 |
| BRN | 878317 |
| Exposure limits | ACGIH: SL .025 mg/100 cm2; Ceiling 0.1 ppb (Skin) |
| Stability: | Stable. Air sensitive. Incompatible with strong oxidizing agents, strong bases. |
| CAS DataBase Reference | 643-79-8(CAS DataBase Reference) |
| NIST Chemistry Reference | O-phthalaldehyde(643-79-8) |
| EPA Substance Registry System | 1,2-Benzenedicarboxaldehyde (643-79-8) |
Safety information for o-Phthalaldehyde
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H314:Skin corrosion/irritation H317:Sensitisation, Skin H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for o-Phthalaldehyde
| InChIKey | ZWLUXSQADUDCSB-UHFFFAOYSA-N |
o-Phthalaldehyde manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 643-79-8 98%View Details
643-79-8 98%View Details
643-79-8 -
![o-Phthalaldehyde [for HPLC Labeling] CAS 643-79-8](https://img.chemicalbook.in//Content/image/CP5.jpg) o-Phthalaldehyde [for HPLC Labeling] CAS 643-79-8View Details
o-Phthalaldehyde [for HPLC Labeling] CAS 643-79-8View Details
643-79-8 -
 o-Phthaldehyde, GR 99%+ CAS 643-79-8View Details
o-Phthaldehyde, GR 99%+ CAS 643-79-8View Details
643-79-8 -
 o-Phthalaldehyde 99% CASView Details
o-Phthalaldehyde 99% CASView Details -
 Phthaldialdehyde CAS 643-79-8View Details
Phthaldialdehyde CAS 643-79-8View Details
643-79-8 -
 o-PHTHALALDEHYDE AR CAS 643-79-8View Details
o-PHTHALALDEHYDE AR CAS 643-79-8View Details
643-79-8 -
 O-phthaladehyde, Seema Biotech, 25 Kg DrumView Details
O-phthaladehyde, Seema Biotech, 25 Kg DrumView Details
643-79-8 -
 O-phthalaldehyde (opa) (CAS Number: 643-79-8)View Details
O-phthalaldehyde (opa) (CAS Number: 643-79-8)View Details
643-79-8
