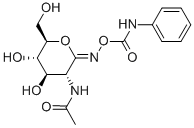
O-[2-ACETAMIDO-2-DEOXY-D-GLUCOPYRANOSYLI
- CAS NO.:132489-69-1
- Empirical Formula: C15H19N3O7
- Molecular Weight: 353.33
- MDL number: MFCD00145022
- EINECS: 1533716-785-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-06 15:28:16
What is O-[2-ACETAMIDO-2-DEOXY-D-GLUCOPYRANOSYLI?
Description
Proteins can be modified post-translationally by the addition of O-linked N-acetylglucosamine (O-GlcNAc). Nuclear cytoplasmic O-GlcNAcase and acetyltransferase (NCOAT) is a β-N-acetylglucosaminidase that removes GlcNAc from O-glycosylated proteins. PUGNAc is a (phenylcarbamoyl)oxime analog of GlcNAc that reversibly inhibits NCOAT (Ki = 40-110 nM). It also less potently inhibits other hexosaminidases and exochitinases. (Z)-PUGNAc is a stereoisomer of PUGNAc that is a more potent inhibitor of NCOAT than the (E) isomer, both in vitro and in cells.
Chemical properties
White to Off-White Solid
The Uses of O-[2-ACETAMIDO-2-DEOXY-D-GLUCOPYRANOSYLI
O-(2-acetamido-2deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc) has been used to evaluate the effects of silibinin on O-GlcNAc levels of glycoproteins in Adult Retinal Pigment Epithelial-19 (ARPE-19) cells. It has also been used as a component of the HEPES lysis buffer for rat brain samples.
The Uses of O-[2-ACETAMIDO-2-DEOXY-D-GLUCOPYRANOSYLI
An inhibitor of O-GlcNAcase, hexosaminidase A, and hexosaminidase B
What are the applications of Application
(Z)-Pugnac is an O-GlcNAcase inhibitor that undergoes Beckmann rearrangement to promote insulin resistance in adipocytes.
Biological Activity
O -GlcNAc- β - N -acetylglucosaminidase ( O -GlcNAcase) and β -hexosaminidase inhibitor (K i values are 46 and 36 nM respectively) that increases O -GlcNAc levels ~ 2-fold in HT29 cells. Z -linked isomer is more potent than the E isomer.
Biochem/physiol Actions
O-(2-acetamido-2deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc) induces insulin resistance in 3T3-L1 adipocytes by reducing insulin-prompting phosphorylation of protein kinase B (Akt) and glycogen synthase kinase 3β (GSK3β).
storage
Desiccate at -20°C
References
1) Macauley et al. (2005), O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors; J. Biol. Chem., 280 25313 2) Kneass and Marchase (2005), Protein O-GlcNAc modulates motility-associated signaling intermediates in neutrophils; J. Biol. Chem., 280 14579 3) Zou et al. (2007), The protective effects of PUGNAC on cardiac function after trauma-hemorrhage are mediated via increased protein O-GlcNAc levels; Shock, 27 402 4) Arias et al. (2004), Prolonged incubation in PUGNAc results in increased protein O-Linked glycosylation and insulin resistance in rat skeletal muscle; Diabetes, 53 921
Properties of O-[2-ACETAMIDO-2-DEOXY-D-GLUCOPYRANOSYLI
| Melting point: | 172-175°C |
| Density | 1.53 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 35 mg/ml) |
| form | solid |
| pka | 11.85±0.70(Predicted) |
| color | White |
| BRN | 4274031 |
| Stability: | Moisture and Temperature Sensitive |
Safety information for O-[2-ACETAMIDO-2-DEOXY-D-GLUCOPYRANOSYLI
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for O-[2-ACETAMIDO-2-DEOXY-D-GLUCOPYRANOSYLI
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 PUGNAc 95% CAS 132489-69-1View Details
PUGNAc 95% CAS 132489-69-1View Details
132489-69-1 -
 PUGNAc CAS 132489-69-1View Details
PUGNAc CAS 132489-69-1View Details
132489-69-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1