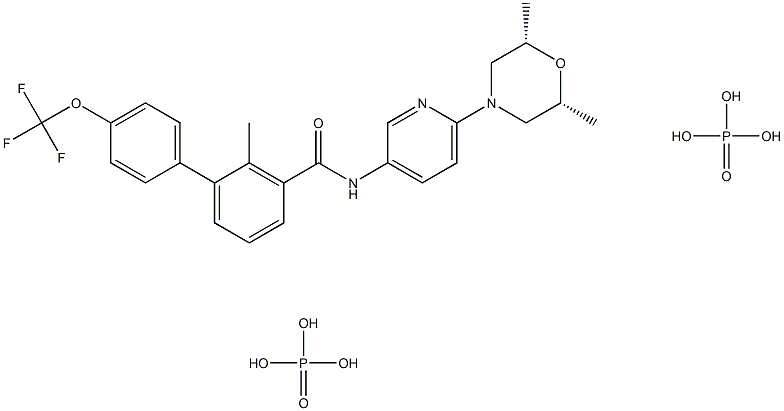
NVP-LDE225
- CAS NO.:956697-53-3
- Empirical Formula: C26H26F3N3O3
- Molecular Weight: 485.5
- MDL number: MFCD16038928
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
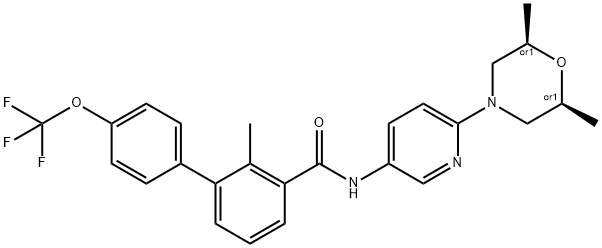
What is NVP-LDE225?
Absorption
Sonidegib is rapidly absorbed in the fasted state with peak concentrations occurring 2-4 hours after administration. (2) However, the total absorption of Sonidegib is low (roughly 6-7%). (1)
Toxicity
Adverse events occurred more frequently with higher doses, 800 mg once daily when compared to a lower dose of 200 mg once daily. In the 200 mg group, frequent adverse events (occurring in ≥2% of patients) included: elevated creatine phosphokinase (6%), increased lipase (5%), muscle spasms (3%), asthenia (3%), and hypertension (3%). In the 800 mg group, frequent adverse events included: elevated creatine phosphokinase (13%), increased lipase (5%), weight loss (5%), muscle spasms (5%), decreased appetite (4%), rhabdomyolysis (3%), nausea (3%), hypertension (3%), increased alanine aminotransferase (3%), increased aspartate aminotransferase (3%), fatigue (2%), syncope (2%), anaemia (2%), dehydration (2%), hyperkalaemia (2%) and myalgia (2%). Rhabdomyolysis cases reported by investigators were not confirmed by the adjudication committee on muscle toxicity or the independent safety review. (2)
The Uses of NVP-LDE225
A potent, selective and orally bioavailable Smoothened (SMO) antagonist; it inhibits hedgehog (Hh) signaling pathway via antagonism of the Smoothened receptor (SMO). Antineoplastic.
The Uses of NVP-LDE225
A potent, selective and orally bioavailable Smoothened (SMO) antagonist; it inhibits hedgehog (Hh) signaling pathway via antagonism of the Smoothened receptor (SMO). Antineoplastic. Potent Hedgehog inhibitor.
Background
Sonidegib is a Hedgehog signaling pathway inhibitor (via smoothened antagonism) developed as an anticancer agent by Novartis. It was FDA approved in 2015 for the treatment of basal cell carcinoma.
Indications
Sonidegib is approved for use in the US and EU for treatment of adults with locally advanced basal cell carcinoma (BCC) that has recurred post surgery or radiation therapy. It is also approved for adult patients with BCC who are not eligible for surgery or radiation therapy. (2)
What are the applications of Application
Erismodegib is a selective Smo (Smoothened) antagonist
Definition
ChEBI: A member of the classo of biphenyls that is the amide obtained by formal condensation of the carboxy group of 2-methyl-4'-(trifluoromethoxy)[1,1'-biphenyl]-3-carboxylic acid with the amino group of 6-(2,6-dimethylmorpholin-4-yl)pyridin-3-amine. Used (as it phosphate salt) for treatment of locally advanced basal cell carcinoma.
Biological Activity
lde225 is a potent and selective inhibitor of smoothened with ic50 values of 1.3nm in mouse and 2.5nm in human, respectively [1].lde225 is screened out from a high-throughput cell-based screen of in-house diversity combinatorial libraries and is developed to be an antagonist of smo. smo is an activator of the hedgehog(hh) signaling pathway and aberrant activation links to tumorigenesis in several cancers. the antitumor efficacy of lde225 has been evaluated in vivo. in the subcutaneous ptch+/-p53-/- medulloblastoma allograft mouse model, lde225 can significantly inhibit tumor growth at a dose of 5mg/kg/day. and in an orthotopic ptch+/-p53-/- medulloblastoma allograft model, lde225 is suggested to penetrate the blood-brain barrier in tumor-bearing animals and cause the tumor growth inhibition after 4 days of treatment. additionally, the preclinical safety assays show that lde225 has no genotoxicity and has good selectivity [1].
Pharmacokinetics
Sonidegib has been shown to inhibit a transmembrane protein called SMO which plays a role in Hh signal transduction. This has resulted in inhibition of Hh signaling as well as antitumour activity in various animal models. In a transgenic mouse model of islet cell neoplasms, tumour volume was reduce by 95% in mice treated with sonidegib when compared with untreated mice. (2)
Metabolism
Sonidegib is primarily metabolized via oxidation and amide hydrolysis. (1) The enzyme responsible for the majority of metabolism is the cytochrome P450 (CYP) 3A4 enzyme. (2)
storage
Store at -20°C
References
[1] shifeng pan, xu wu, jiqing jiang, et al. discovery of nvp-lde225, a potent and selective smoothened antagonist. acs med. chem. lett. 2010, 1: 130–134.
Properties of NVP-LDE225
| Melting point: | 154-157°C |
| Boiling point: | 544.5±50.0 °C(Predicted) |
| Density | 1.255 |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | White solid. |
| pka | 9.53±0.70(Predicted) |
| color | Light Purple |
Safety information for NVP-LDE225
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for NVP-LDE225
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![N-[3-(1H-Benzimidazol-2-yl)-4-chlorophenyl]-3,4,5-triethoxybenzamide](https://img.chemicalbook.in/CAS/GIF/329196-48-7.gif)
You may like
-
 Nvp-lde225 95% CAS 956697-53-3View Details
Nvp-lde225 95% CAS 956697-53-3View Details
956697-53-3 -
 Erismodegib 98% (HPLC) CAS 956697-53-3View Details
Erismodegib 98% (HPLC) CAS 956697-53-3View Details
956697-53-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1