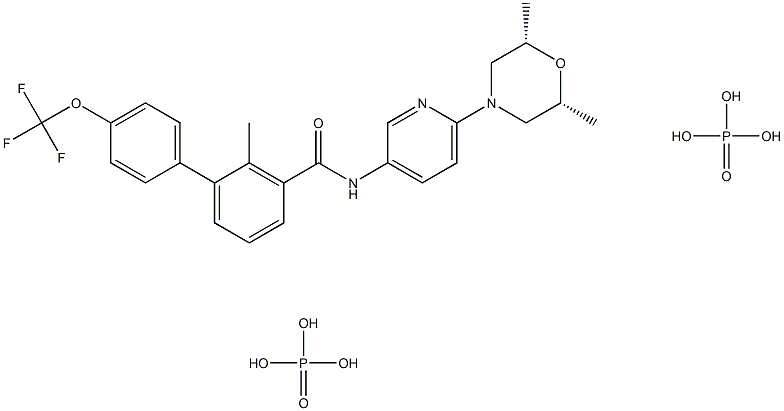
LDE-225 Diphosphate
- CAS NO.:1218778-77-8
- Empirical Formula: C26H32F3N3O11P2
- Molecular Weight: 681.4885116
- MDL number: MFCD16627994
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is LDE-225 Diphosphate?
Description
Sonidegib phosphate (XXIX), an orally bioavailable, small molecule smoothened (SMO) receptor antagonist developed by Novartis, was approved in 2015 in Switzerland, the U.S., and the EU for the treatment of adult patients with advanced or locally advanced basal cell carcinoma (BCC). BCC is the most frequently diagnosed skin cancer, constituting 80% of all nonmelanoma skin cancers. While most BCCs can be treated through surgery or radiation therapy, some patients (<10%) have more advanced tumors for which surgery may be contraindicated or impractical; treatment options for these patients are limited. SMO, a G-protein coupled receptor-like protein (GPCR), is a key regulator in the hedgehog (Hh) pathway, which is activated in a number of tumors including BCC. In a multicenter clinical trial for sonidegib, an objective response rate of 43% was observed for patients with locally advanced BCC (dosing at 200 mg once daily), with sustained clinically meaningful responses based on an 18-month analysis. Sonidegib joins vismodegib as marketable treatments of BCC. Vismodegib, which was approved in 2012 as a first-in-class SMO receptor antagonist, represented the first Hedgehog signaling pathway targeting agent to gain FDA approval.
The Uses of LDE-225 Diphosphate
NVP-LDE225 Diphosphate Salt is a potent, selective, and orally bioavailable Smoothened (Hedgehog Signaling Pathway) antagonist.
Definition
ChEBI: A phosphate salt obtained by reaction sonidegib with two equivalent of phosphoric acid. Used for treatment of locally advanced basal cell carcinoma.
Synthesis
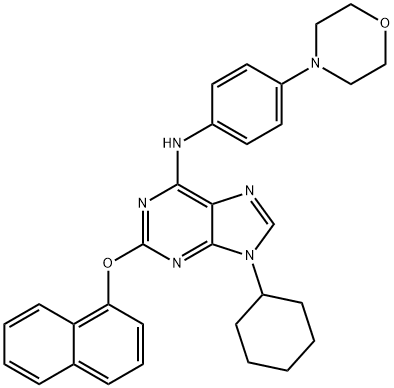
The synthesis was initiated with an SNAr reaction involving commercial 2-chloro- 5-nitropyridine (226) and cis-dimethylmorpholine (227) followed by subsequent nitro reduction via hydrogenation to provide amine 228. The crude amine was coupled directly to 3- bromo-2-methylbenzoic acid (229) in an amide bond-forming reaction to construct 230 in 77% yield over three steps. The resultant bromide was coupled to 4-(trifluoromethoxy)- phenylboronic acid (231) under Suzuki conditions to give rise to sonidegib as the freebase. Finally, treatment with 85% aqueous phosphoric acid in acetonitrile generated sonidegib phosphate (XXIX) in good yield.
in vitro
lde225 was found to selectively bind to the hedgehog (hh)-ligand cell surface receptor smo, which might result in the suppression of the hh signaling pathway and, therefore, the inhibition of tumor cells in which this pathway was abnormally activated [1].
in vivo
in the subcutaneous medulloblastoma allograft mouse model, lde225 demonstrated dose-related antitumor activity after 10 days of oral administration of a suspension. at a dose of 5 mg/kg/ day qd, lde225 inhibited tumor growth significantly, corresponding to a t/c value of 33%. when dosed at 10 and 20 mg/kg/day qd, lde225 afforded 51 and 83% regression, respectively [1].
References
[1] shifeng pan,xu wu,jiqing jiang et al. discovery of nvp-lde225, a potent and selective smoothened antagonist. acs med chem lett. 2010 jun 10; 1(3): 130–134.
[2] rodon j,tawbi ha,thomas al,stoller rg,turtschi cp,baselga j,sarantopoulos j,mahalingam d,shou y,moles ma,yang l,granvil c,hurh e,rose kl,amakye dd,dummer r,mita ac. a phase i, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor sonidegib (lde225) in patients with advanced solid tumors. clin cancer res.2014 apr 1;20(7):1900-9.
Properties of LDE-225 Diphosphate
| storage temp. | Store at -20°C |
| solubility | ≥27.85 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
| form | White solid. |
| color | White to off-white |
Safety information for LDE-225 Diphosphate
Computed Descriptors for LDE-225 Diphosphate
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
![N-[3-(1H-Benzimidazol-2-yl)-4-chlorophenyl]-3,4,5-triethoxybenzamide](https://img.chemicalbook.in/CAS/GIF/329196-48-7.gif)
You may like
-
 NVP-LDE225 Diphosphate salt >95% CAS 1218778-77-8View Details
NVP-LDE225 Diphosphate salt >95% CAS 1218778-77-8View Details
1218778-77-8 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4