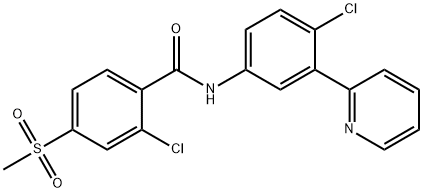
Vismodegib
- CAS NO.:879085-55-9
- Empirical Formula: C19H14Cl2N2O3S
- Molecular Weight: 421.3
- MDL number: MFCD12407408
- EINECS: 806-752-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
What is Vismodegib?
Absorption
Vismodegib appears to have a nonlinear pharmacokinetic profile following daily oral dosing, and steady state is achieved within 7 days. A dose increase from 150 mg to 540 mg (1 to 3.6 times the recommended dose) does not lead to an increase in steady-state plasma concentrations. With a once-daily dose of 150 mg, the average plasma concentration of vismodegib at steady state is approximately 23 μM. The absolute bioavailability of a single dose of vismodegib is 31.8%. Absorption is saturable and is not affected by food.
Toxicity
Toxicity information regarding vismodegib is not readily available. Patients experiencing an overdose are at an increased risk of severe adverse effects such as severe cutaneous adverse reactions and musculoskeletal adverse reactions. Symptomatic and supportive measures are recommended. Patients treated with vismodegib have an increased risk of embryo-fetal death and significant birth defects. Common adverse event include muscle spasms, alopecia, dysgeusia, weight loss, fatigue, nausea, diarrhea, decreased appetite, constipation, arthralgias, vomiting, and ageusia.
Based on the results of in vitro and in vivo studies, vismodegib is not mutagenic. No evidence of carcinogenicity was found in mice and rats given vismodegib. A 26-week rat fertility study found that at doses of 100 mg/kg/day, vismodegib has no effects on male reproductive organs or fertility. In female rats, the administration of vismodegib was associated with decreased implantations, increased percent preimplantation loss, and decreased numbers of dams with viable embryos.
Description
In January 2012, the US FDA approved vismodegib (also referred to as GDC-0449) for the treatment of adults with metastatic basal cell carcinoma (BCC), with locally advanced BCC that has recurred following surgery or who are not candidates for surgery or radiation. Vismodegib inhibits the Hh sig?naling pathway by functioning as an antagonist of SMO thereby inhibiting the activation of Hedgehog target genes, resulting in decreased downstream pro?duction of proliferation factors. The IC50 of vismodegib in a Hedgehog?responsive cell line derived from human embryonic palatal mesenchyme cells was 2.8 nM. In preclinical in vivostudies, vismodegib at 12.5 mg/kg (bid) caused complete regression of tumors in a Hh pathway dependent medulloblas?tomaallograft model generated from Ptch+/-mice. A synthesis of vismodegib starting from 2-chloro-5-nitro aniline and employing a Negishi coupling with 2-pyridyl zinc iodide as a key step has been reported.
Description
Vismodegib is a drug that the US Food and Drug Administration approved in 2012 for treating basal-cell carcinoma, a sometimes difficult-to-eradicate skin cancer. It is marketed by Genentech (South San Francisco, CA) under the trade name Erivedge.
Vismodegib is the first drug to be approved by FDA that targets the hedgehog* (Hh) signaling pathway. The?Hhpathway is vital for the formation of organs in the body, and abnormal?Hh?signaling is associated with the development of cancer cells.
This past October, the Genentech research team received one of six?ACS 2017 Heroes of Chemistry awards?for developing products that improve people’s lives.
Genentech has more plans for vismodegib. It is in clinical trials for other cancers, including pancreatic, stomach, and lung.
*Why hedgehog? It’s the name of a signaling molecule that is encoded by the?Hh?gene. The absence of the?gene makes fruit fly larvae look like the bristly animal of the same name.
Chemical properties
White Solid
Originator
Curis/Genentech (United States)
The Uses of Vismodegib
Targets the Hedgehog (Hh) pathway. Inhibition of the Hh signaling may be effective in the treatment and prevention of many types of human cancers.
The Uses of Vismodegib
Vismodegib (GDC-0449) is a potent, novel and specific hedgehog inhibitor with IC50 of 3 nM and also inhibits P-gp with IC50 of 3.0 μM.
The Uses of Vismodegib
Vismodegib targets the Hedgehog (Hh) pathway. Inhibition of the Hh signaling may be effective in the treatment and prevention of many types of human cancers. Potent Hedgehog inhibitor.
What are the applications of Application
Vismodegib is a potent hedgehog (Hh) signaling pathway inhibitor
Background
Vismodegib is an orally active small molecule that inhibits the hedgehog signaling pathway by binding to and inhibiting the transmembrane protein smoothened homologue (SMO). It was discovered by high-throughput screening of a library of small-molecule compounds and subsequent optimization through medicinal chemistry. Since it targets the hedgehog signaling pathway, vismodegib has anti-tumor activity in basal-cell carcinoma. The Hedgehog signaling pathway plays an important role in the development of organs and tissues during embryogenesis. Afterwards, it is silenced in all cells and tissues, except for hair, skin and stem cells. However, dysregulated or aberrant Hedgehog signaling has been associated with basal cell carcinoma pathogenesis. In January 2012, vismodegib was approved by the FDA for the treatment of adult basal cell carcinoma. In July 2013, it was approved by the EMA, and since then, it has been approved by several other countries.
Indications
Vismodegib is indicated for the treatment of adults with metastatic basal cell carcinoma, or with locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates for surgery and who are not candidates for radiation.
Definition
ChEBI: A benzamide obtained by formal condensation between the carboxy group of 2-chloro-4-(methylsulfonyl)benzoic acid and the anilino group of 4-chloro-3-(pyridin-2-yl)aniline. Used for the treatment metastatic basal cell carcinoma.
brand name
Erivedge
Pharmacokinetics
Vismodegib selectively binds to and inhibits the transmembrane protein smoothened homologue (SMO) to inhibit the Hedgehog signalling pathway. Following 7 days of 150 mg once-daily dosing, the use of vismodegib was not associated with a clinically significant QT interval prolongation. Vismodegib can cause embryo-fetal death or severe birth defects, as well as severe cutaneous adverse reactions and musculoskeletal adverse reactions. In pediatric patients given vismodegib, premature fusion of the epiphyses has been reported.
Clinical Use
Antineoplastic agent:
Treatment of basal cell carcinoma which is inappropriate for surgery or radiotherapy
Synthesis
The synthesis began with selective iodination of commercial carboxylic acid 179, affording trisubstituted arene 180 in 73% yield. A Curtius reaction then converted 180 to carbamate 181 in 84% 52 yield, and this was followed by a palladium(0)-catalyzed borylation of 181 which furnished Suzuki coupling partner 182 in 91% yield. Pinacol borane 182 was exposed to commercial 2-bromopyridine under conventional cross-coupling conditions to furnish biaryl 183, which underwent Boc-deprotection in quantitative conversion to generate 184. Amide bond formation with acid chloride 185 (readily available from the corresponding commercial acid) produced vismodegib (XXVIII) in 99% yield.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration possibly reduced by
rifampicin - avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration possibly reduced by
carbamazepine, fosphenytoin and phenytoin - avoid.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Metabolism
Vismodegib is mainly metabolized by CYP2C9 and CYP3A4 in the liver; however, more than 98% of total systemic vismodegib is not metabolized. Metabolic pathways of vismodegib in humans include oxidation, glucuronidation, and pyridine ring cleavage. The two most abundant oxidative metabolites recovered in feces are produced in vitro by recombinant CYP2C9 and CYP3A4/5.
Metabolism
Vismodegib is hepatically metabolised by CYP2C9 and
CYP3A4, however more than 98
% of total systemic
vismodegib is not metabolised. Metabolic pathways
of vismodegib include oxidation, glucuronidation, and
pyridine ring cleavage. The two most abundant oxidative
metabolites recovered in faeces are produced in vitro by
recombinant CYP2C9 and CYP3A4/5.
Vismodegib is slowly eliminated by a combination of
metabolism and excretion of parent drug, the majority
is recovered in the faeces (82
%). Vismodegib and its
metabolites are eliminated mainly by the hepatic route.
Storage
Store at -20°C
References
1) Rominger et al. (2009), Evidence for allosteric interactions of antagonist binding to the smoothened receptor; J. Pharmacol. Exp. Therap., 329 995 2) Tian et al. (2012), The hedgehog pathway inhibitor GDC-0449 alters intracellular Ca2+ homeostasis and inhibits cell growth in cisplatin-resistant lung cancer cells; Anticancer Res., 32 89 3) Zhang et al. (2009), Hedgehog pathway inhibitor HhAntag691 is a potent inhibitor of ABCG2/BCRP and ABCB1/Pgp; Neoplasia, 11 96 4) Cirrone and Harris (2012), Vismodegib anf the hedgehog pathway: a new treatment for basal cell carcinoma; Clin. Ther., 34 2039 5) Wu et al. (2017), Smoothened antagonist GDC-0449 (Vismodegib) inhibits proliferation and triggers apoptosis in colon cancer cell lines; Exp. Ther. Med., 13 2529 6)Singh et al. (2011) Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms; PLoS One 6?e27306 7) Rudin et al. (2009) Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449; N. Engl. J. Med., 361?1173 8) Von Hoff et al. (2009) Inhibition of the hedgehog pathway in advanced basal-cell carcinoma; N. Engl. J. Med., 361?1164
Properties of Vismodegib
| Melting point: | 179-181°C |
| Boiling point: | 561.6±50.0 °C(Predicted) |
| Density | 1.440 |
| storage temp. | -20° |
| solubility | Soluble in DMSO (up to 200 mg/ml) or in Ethanol (up to 10 mg/ml with warming) |
| form | White solid. |
| appearance | white crystals |
| pka | 10.72±0.70(Predicted) |
| color | Off-white or beige |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
Safety information for Vismodegib
Computed Descriptors for Vismodegib
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 879085-55-9 Vismodegib 98%View Details
879085-55-9 Vismodegib 98%View Details
879085-55-9 -
 Vismodegib 99% (HPLC) CAS 879085-55-9View Details
Vismodegib 99% (HPLC) CAS 879085-55-9View Details
879085-55-9 -
 Vismodegib 98% CAS 879085-55-9View Details
Vismodegib 98% CAS 879085-55-9View Details
879085-55-9 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
