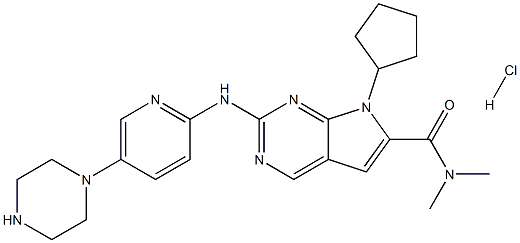
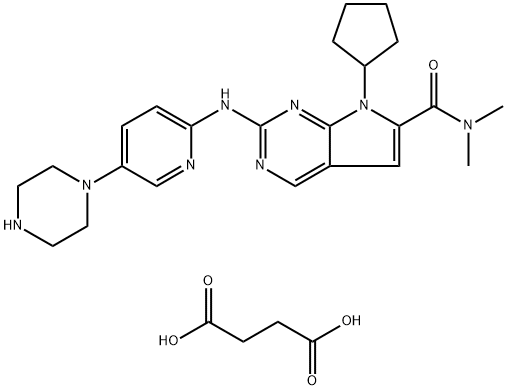
Ribociclib
- CAS NO.:1211441-98-3
- Empirical Formula: C23H30N8O
- Molecular Weight: 434.54
- MDL number: MFCD27976795
- EINECS: 807-111-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
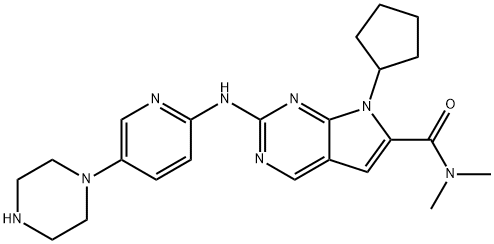
What is Ribociclib?
Absorption
Ribociclib is orally bioavailable, highly selective inhibitor of CDK4/6 kinases with inhibitory IC50 concentrations in the low nanomolar range. Following oral dosing, ribociclib was rapidly absorbed with median Tmax ranging from 1 to 5 hours. Plasma concentrations increased approximately 2- to 3-fold from Cycle 1 Day 1 to Cycle 1 Day 18/21 due to accumulation, with steady state reached by approximately Day 8 on the basis of trough concentrations after repeated daily dosing. Dose-proportionality analyses demonstrated that exposure to ribociclib increased with dose, with both Cmax and area under the curve (AUC) increasing slightly more than proportional to dose, over the dose range 50–1,200 mg/day
Description
LEE011 is a cyclin-
Characteristics
Class: serine/threonine protein kinase
Treatment: Breast cancer
Oral bioavailability = 66%
Elimination half-life = 32 h
Protein binding = 70%
The Uses of Ribociclib
LEE 011 can be used in biological study of kinome-wide RNA interference screen reveals a role for PDK1 in acquired resistance to CDK4/6 inhibition in ER-positive breast cancer.
Background
Ribociclib is a selective cyclin-dependent kinase inhibitor, a class of drugs that help slow the progression of cancer by inhibiting two proteins called cyclin-dependent kinase 4 and 6 (CDK4/6). These proteins, when over-activated, can enable cancer cells to grow and divide too quickly. Targeting CDK4/6 with enhanced precision may play a role in ensuring that cancer cells do not continue to replicate uncontrollably. Ribociclib was approved by the U.S. FDA in March, 2017 as Kisqali.
Indications
Kisqali (ribociclib) is a selective cyclin-dependent kinase inhibitor, a class of drugs that help slow the progression of cancer by inhibiting two proteins called cyclin-dependent kinase 4 and 6 (CDK4/6). These proteins, when over-activated, can enable cancer cells to grow and divide too quickly. Targeting CDK4/6 with enhanced precision may play a role in ensuring that cancer cells do not continue to replicate uncontrollably.
Mechanism of action
Ribociclib is a dual cdk4/cdk6 inhibitor. Induces G1 phase cell cycle arrest and senescence in neuroblastoma cell lines (IC50 = 306 nM). Delays tumor growth in vivo.
Clinical Use
Protein kinase inhibitor:
Treatment of breast cancer
Clinical Use
Ribociclib was first approved in 2017 as the second CDK4/6 (cyclin-dependent kinase) inhibitor in combination with aromatase inhibitor as initial endocrine-based therapy for the treatment of postmenopausal women with hormone receptor positive (HR+), human epidermal growth factor receptor-2 negative (HER2?) advanced or metastatic breast cancer. In 2018, its label was expanded to pre/perimenopausal women with HR+/HER2? advanced or metastatic breast cancer.
Drug interactions
Potentially hazardous interactions with other drugs
The concomitant use of strong CYP3A4 inhibitors
including, but not limited to, the following must
be avoided: clarithromycin, indinavir, itraconazole,
ketoconazole, lopinavir, ritonavir, nefazodone,
nelfinavir, posaconazole, saquinavir, telaprevir,
telithromycin, verapamil and voriconazole.
The concomitant use of strong CYP3A4 inducers
may therefore lead to decreased exposure and
consequently a risk for lack of efficacy. The
concomitant use of strong CYP3A4 inducers should
be avoided, including, but not limited to, phenytoin,
rifampicin, carbamazepine and St John's wort.
Caution and monitoring for toxicity are advised
during concomitant treatment with sensitive
substrates of drug transporters P-gp, BCRP,
OATP1B1/1B3, OCT1, OCT2, MATE1 and
BSEP which exhibit a narrow therapeutic index,
including but not limited to digoxin, pravastatin,
rosuvastatin and metformin.
Co-administration of Kisqali with medicinal
products with a known potential to prolong the
QT interval such as anti-arrhythmic medicinal
products (including, but not limited to, amiodarone,
disopyramide, procainamide, quinidine and sotalol),
and other medicinal products that are known to
prolong the QT interval (including, but not limited
to, chloroquine, halofantrine, clarithromycin,
haloperidol, methadone, moxifloxacin, pimozide and
intravenous ondansetron) should be avoided.
Side Effects
The most common side effects of ribociclib include infections, low levels of white blood cells, headache, cough, nausea (feeling sick), vomiting, diarrhoea, constipation, tiredness, hair loss and rash.The most common severe side effects include infections, low levels of red and white blood cells, vomiting, abnormal blood tests for liver function and low levels of phosphate in the blood (hypophosphataemia).
Metabolism
Ribociclib is hepatically metabolised via CYP3A4 by
oxidation. Ribociclib was the main circulating drug in
plasma (44%). The major circulating metabolites included
metabolite M13 (CCI284, N-hydroxylation), M4 (LEQ803,
N-demethylation), and M1 (secondary glucuronide).
Clinical activity of ribociclib was due mainly to parent drug,
with negligible contribution from circulating metabolites.
Unchanged drug accounted for 17.3% and 12.1% of
the dose in faeces and urine, respectively. Metabolite
LEQ803 represented approximately 13.9% and 3.74%
of the administered dose in faeces and urine, respectively.
Numerous other metabolites were detected in both faeces
and urine in minor amounts (≤2.78%).
Ribociclib and its metabolites are eliminated mainly via
faeces (69.1%), with a small contribution from the renal
route (22.6%).
Storage
Store at -20°C
References
1) Rader?et al.?(2013),?Dual CDK4/6 inhibition induces cell-cycle arrest and senescence in neuroblastoma; Clin. Cancer Res.?19?6173 2) Kim?et al. (2013),?LEE011: an orally bioavailable, selective small-molecule inhibitor of CDK4/6 – reactivating Rb in cancer; Mol. Cancer Ther.?12?PR02 3) Tripathy?et al.?(2017),?Ribociclib(LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors; Clin. Cancer Res. March 28, 201
Properties of Ribociclib
| Boiling point: | 730.8±70.0 °C(Predicted) |
| Density | 1.39±0.1 g/cm3(Predicted) |
| vapor pressure | 0-0Pa at 20-25℃ |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 10 mg/ml) |
| form | solid |
| pka | 8.67±0.10(Predicted) |
| color | Off-white |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months. |
Safety information for Ribociclib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ribociclib
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 1211441-98-3 Ribociclib 98%View Details
1211441-98-3 Ribociclib 98%View Details
1211441-98-3 -
 Ribociclib 97%View Details
Ribociclib 97%View Details -
 Ribociclib 98%View Details
Ribociclib 98%View Details
1211441-98-3 -
 Lee011 95% CAS 1211441-98-3View Details
Lee011 95% CAS 1211441-98-3View Details
1211441-98-3 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
