Nonanoic acid
Synonym(s):Acid C9;Nonanoic acid;Pelargonic acid
- CAS NO.:112-05-0
- Empirical Formula: C9H18O2
- Molecular Weight: 158.24
- MDL number: MFCD00004433
- EINECS: 203-931-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
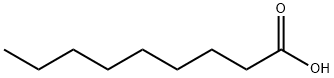
What is Nonanoic acid?
Description
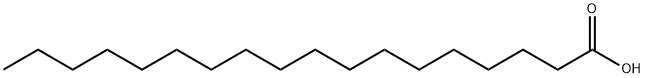
Nonanoic acid , also called pelargonic acid, is an organic compound composed of a nine - carbon chain terminating in a carboxylic acid with structural formula CH3(CH2)7COOH. Nonanoic acid forms esters—nonanoates. It is a clear, oily liquid with an unpleasant, rancid odor. It is nearly insoluble in water, but very soluble in chloroform, ether, and hexane.
Its refractive index is 1.4322. Its critical point is at 712 K ( 439 °C ) and 2.35 MPa.
Chemical properties
clear colorless liquid
Chemical properties
Colorless or yellowish, combustible, oily liquid. Faint odor.
Chemical properties
Nonanoic acid has a fatty, characteristic odor and a corresponding unpleasant taste. May be prepared by oxidation of methylnonyl ketone; by oxidation of oleic acid; or from heptyl iodide via malonic ester synthesis.
Chemical properties
Nonanoic acid has a fatty, characteristic odor and a corresponding unpleasant taste. This compound is also reported as having a cheese, waxy flavor
Originator
Pellar,Crookes Barnes, US ,1960
Occurrence
Nonanoic acid is a fatty acid which occurs naturally as esters in the oil of pelargonium. Synthetic esters, such as methyl nonanoate, are used as flavorings.
Nonanoic acid is also used in the preparation of plasticizers and lacquers.
The derivative 4-nonanoylmorpholine is an ingredient in some pepper sprays.
The ammonium salt of nonanoic acid, ammonium nonanoate, is used as an herbicide.
The Uses of Nonanoic acid
The primary uses of this acid are in organic synthesis and in the manufacture of lacquers, plastics, pharmaceuticals, synthetic odors and flavorings, gasoline additives, flotation agents, lubricants, and vinyl plasticizers. Nonanoic acid has found some use in pharmaceutical preparations and as a topical bactericide and fungicide. It is also used in herbicides.
The Uses of Nonanoic acid
Intermediates of Liquid Crystals
The Uses of Nonanoic acid
In the production of hydrotropic salts (hydrotropic salts form aqueous solutions which dissolve sparingly soluble substances to a greater extent than water); in the manufacture of lacquers, plastics.
What are the applications of Application
Nonanoic acid is a lipid used in the preparation of plasticizers and lacquers
Definition
ChEBI: A C9 straight-chain saturated fatty acid which occurs naturally as esters of the oil of pelargonium. Has antifungal properties, and is also used as a herbicide as well as in the preparation of plasticisers and lacquers.
Preparation
Nonanoic acid is produced by malonic ester synthesis, from Heptyl iodide.
Manufacturing Process
A body of liquid, 18 inches high, comprising a 35% (by weight) solution of technical (95%) oleic acid in n-propanol, is maintained at a temperature of 86°C in a reactor. The solution also contains dissolved therein 0.042% by weight of cobalt, in the form of cobalt naphthenate. From the bottom of the reactor very fine bubbles of air are passed into and through the solution at the rate of about 0.3 cubic feet per minute, measured at standard conditions, per square foot for 72 hours. The gases leaving the reactor are first passed through an ice water reflux condenser and then vented to the atmosphere. At the end of the 72 hour period the reaction mixture is separated into its components. It is found that 60% of the oleic acid has been consumed in the reaction. For each pound of oleic acid consumed there are obtained 0.30 pound of azelaic acid (representing an efficiency of 46%, calculated on the basis that the technical oleic acid is 100% oleic acid), 0.13 pound of pelargonic acid (representing an efficiency of 23%) and 0.21 pound of 9,10dihydroxystearic acid (representing an efficiency of 19%).
Therapeutic Function
Fungicide
Aroma threshold values
Detection: 3 to 9 ppm
Taste threshold values
Taste characteristics at 10 ppm: fatty, waxy, and cheesy with a mild, sweet, creamy background
Synthesis Reference(s)
Journal of the American Chemical Society, 93, p. 195, 1971 DOI: 10.1021/ja00730a033
Organic Syntheses, Coll. Vol. 2, p. 474, 1943
Tetrahedron Letters, 9, p. 5689, 1968
General Description
Nonanoic acid occurs naturally in Pelargonium L′Her. It one of the flavor constituents of cooked rice, acerola fruit, licorice and yogurt.
Agricultural Uses
Herbicide, Fungicide: Pelargonic acid occurs naturally in many plants and animals. It is used to control the growth of weeds and as a blossom thinner for apple and pear trees. It is also used as a food additive; as an ingredient in solutions used to commercially peel fruits and vegetables
Trade name
CIRRASOL®-185A; ECONOSAN®; EMERY® 202 (mixture with n-octoic acid); EMFAC®-1202; HEXACID® C-9; PELARGON®; SCYTHE®; WEST AGRO ACID SANITIZER®
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion. A severe skin and eye irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
By oxidation of methylnonyl ketone; by oxidation of oleic acid; or from heptyl iodide via malonic ester synthesis
Potential Exposure
Pelargonic acid, a naturally occurring fatty acid herbicide/fungicide. It is used to control the growth of weeds and as a blossom thinner for apple and pear trees. It is also used as a food additive; as an ingredient in solutions used to commercially peel fruits and vegetables; in the manufacture of lacquers, plastics and pharmaceuticals.
Purification Methods
Esterify the acid with ethylene glycol and distil the ester. (This removes dibasic acids as undistillable residues.) The acid is regenerated by hydrolysing the ester in the usual way and is distilled in vacuo. [Beilstein 2 IV 1018.]
Incompatibilities
Heated vapors may form explosive mixture with air. May react violently with strong oxidizers, bromine, 90% hydrogen peroxide, phosphorus trichloride, silver powders or dust. Incompatible with strong bases and silver compounds; mixture with some silver compounds may form explosive salts of silver oxalate.
Waste Disposal
Do not discharge into drains or sewers. Dispose of waste material as hazardous waste using a licensed disposal contractor to an approved landfill. Contact a licensed disposal facility about surplus and non-recyclable solutions. Burn in a chemical incinerator equipped with an afterburner and scrubber. Extra care must be exercised as the material in an organic solvent is highly flammable. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Incineration with effluent gas scrubbing is recommended. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of Nonanoic acid
| Melting point: | 9 °C (lit.) |
| Boiling point: | 268-269 °C (lit.) |
| Density | 0.906 g/mL at 25 °C (lit.) |
| vapor density | 5.5 (vs air) |
| vapor pressure | <0.1 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 2784 | NONANOIC ACID |
| Flash point: | 212 °F |
| storage temp. | 2-8°C |
| solubility | 0.3g/l |
| form | Liquid |
| pka | 4.96(at 25℃) |
| color | Clear colorless |
| PH | 4(1 mM solution);3.49(10 mM solution);2.98(100 mM solution); |
| Odor | at 10.00 % in dipropylene glycol. waxy dirty cheese cultured dairy |
| explosive limit | 0.8-9%(V) |
| Water Solubility | NEGLIGIBLE |
| Merck | 14,7070 |
| JECFA Number | 102 |
| BRN | 1752351 |
| Dielectric constant | 13.9(25℃) |
| CAS DataBase Reference | 112-05-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Nonanoic acid(112-05-0) |
| EPA Substance Registry System | Nonanoic acid (112-05-0) |
Safety information for Nonanoic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
Computed Descriptors for Nonanoic acid
Nonanoic acid manufacturer
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Pelargonic Acid 98%View Details
Pelargonic Acid 98%View Details -
 Nonanoic Acid CAS 112-05-0View Details
Nonanoic Acid CAS 112-05-0View Details
112-05-0 -
 n-Nonanoic acid CAS 112-05-0View Details
n-Nonanoic acid CAS 112-05-0View Details
112-05-0 -
 Nonanoic acid 95% CAS 112-05-0View Details
Nonanoic acid 95% CAS 112-05-0View Details
112-05-0 -
 Pelargonic acid CAS 112-05-0View Details
Pelargonic acid CAS 112-05-0View Details
112-05-0 -
 Nonanoic acid CAS 112-05-0View Details
Nonanoic acid CAS 112-05-0View Details
112-05-0 -
 Nonanoic acid CAS 112-05-0View Details
Nonanoic acid CAS 112-05-0View Details
112-05-0 -
 Pelargonic Acid, LiquidView Details
Pelargonic Acid, LiquidView Details
112-05-0
