Decanoic acid
Synonym(s):Decanoic acid;Capric acid;C10:0;1-Nonanecarboxylic acid;Acid C10
- CAS NO.:334-48-5
- Empirical Formula: C10H20O2
- Molecular Weight: 172.26
- MDL number: MFCD00004441
- EINECS: 206-376-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
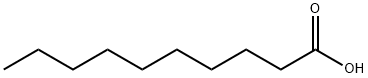
What is Decanoic acid?
Description
Decanoic acid, or capric acid, is a saturated fatty acid. Its formula is CH3(CH2)8COOH. Salts and esters of decanoic acid are called decanoates or "caprates". The term capric acid arises from the Latin "capric" which pertains to goats due to their olfactory similarities.
Capric acid occurs naturally in coconut oil (about 10%) and palm kernel oil (about 4 %), otherwise it is uncommon in typical seed oils. It is found in the milk of various mammals and to a lesser extent in other animal fats.
Two other acids are named after goats: caproic (a C6 fatty acid) and caprylic (a C8 fatty acid). Along with decanoic acid, these total 15 % in goat milk fat.
Chemical properties
White crystalline solid or needles. Fatty, unpleasant, rancid odor.
Occurrence
Reported found in apple, beer, preferments of bread, butter, oil, cheese, blue cheese, Romano cheese, cheddar cheese, Roquefort cheese, roasted cocoa bean, cognac, muscat grape, grape musts and wine, and other natural sources. Also reported in citrus peel oils, orange juice, apricots, guava, papaya, strawberry, butter, yogurt, milk, mutton, hop oil, Bourbon and Scotch whiskey, rum, coffee, mango and tea.
The Uses of Decanoic acid
Decanoic acid is used in manufacturing of esters for artificial fruit flavors and perfumes.
The Uses of Decanoic acid
Intermediates of Liquid Crystals
The Uses of Decanoic acid
manufacture of esters for artificial fruit flavors and perfumes; as an intermediate in other chemical syntheses.
The Uses of Decanoic acid
Manufacturing of esters for artificial fruit flavors and perfumes. Also as an intermediate in chemical syntheses. It is used in organic synthesis and industrially in the manufacture of perfumes, lubricants, greases, rubber, dyes, plastics, food additives and pharmaceuticals.
Pharmaceuticals
Decanoate salts and esters of various drugs are available. Since decanoic acid is a fatty acid, forming a salt or ester with a drug will increase its lipophilicity and its affinity for fatty tissue. Since distribution of a drug from fatty tissue is usually slow, one may develop a long-acting injectable form of a drug (called a Depot injection) by using its decanoate form. Some examples of drugs available as a decanoate ester or salt include nandrolone, fluphenazine, bromperidol, haloperidol and vanoxerine.
What are the applications of Application
Decanoic acid is a lipid used in manufacturing of esters
Definition
ChEBI: Decanoic acid is a C10, straight-chain saturated fatty acid. It has a role as an antibacterial agent, an anti-inflammatory agent, a human metabolite, a volatile oil component, a plant metabolite and an algal metabolite. It is a straight-chain saturated fatty acid and a medium-chain fatty acid. It is a conjugate acid of a decanoate. It derives from a hydride of a decane.
Production Methods
Decanoic acid can be prepared from oxidation of primary alcohol decanol, by using chromium trioxide (CrO3) oxidant under acidic conditions.
Neutralization of decanoic acid or saponification of its esters, typically triglycerides, with sodium hydroxide will give sodium decanoate. This salt (CH3(CH2)8COO-Na+) is a component of some types of soap.
Preparation
Decanoic acid is prepared from Coconut oil by saponification.
Aroma threshold values
Detection: 2.2 to 102 ppm
Synthesis Reference(s)
Synthetic Communications, 20, p. 1617, 1990 DOI: 10.1080/00397919008053081
Synthesis, p. 99, 1970
General Description
White crystalline solid with a rancid odor. Melting point 31.5°C. Soluble in most organic solvents and in dilute nitric acid; non-toxic. Used to make esters for perfumes and fruit flavors and as an intermediate for food-grade additives.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Capric acid reacts exothermically to neutralize bases. Can react with active metals to form gaseous hydrogen and a metal salt. May absorb enough water from the air and dissolve sufficiently in Capric acid to corrode or dissolve iron, steel, and aluminum parts and containers. Reacts with cyanide salts or solutions of cyanide salts to generate gaseous hydrogen cyanide. Reacts exothermically with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides to generate flammable and/or toxic gases. Can react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Reacts with carbonates and bicarbonates to generate a harmless gas (carbon dioxide). Can be oxidized exothermically by strong oxidizing agents and reduced by strong reducing agents; a wide variety of products is possible. May initiate polymerization reactions or catalyze (increase the rate of) reactions among other materials.
Health Hazard
Harmful if swallowed or inhaled. Material is irritating to tissues of mucous membranes, and upper respiratory tract, eyes and skin.
Fire Hazard
Capric acid is combustible.
Flammability and Explosibility
Not classified
Biochem/physiol Actions
Decanoic acid is helpful in the attenuation of oxidative stress. Decanoic acid in ketogenic diet is involved in mitochondrial biogenesis thereby enhancing the citrate synthase and complex I activity of electron transport chain.
Safety Profile
Poison by intravenous route. Mutation data reported. A moderate skin irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
Deconoic acid (fatty acids, saturated, linear, number of C-atoms ≥8 and ≤12, with termi- nating carboxyl group) is a carboxylic acid microbiocide used in cleaning, sanitizing and disinfecting applications for food processors and dairy farmers.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
The acid is best purified by conversion into its methyl ester, b 114.0o/15mm (using excess MeOH, in the presence of H2SO4). The H2SO4 and MeOH are removed, the ester is distilled in vacuo through a 3ft column packed with glass helices. The acid is then obtained from the ester by saponification and vacuum distillation. [Trachtman & Miller J Am Chem Soc 84 4828 1962, Beilstein 2 IV 1041.]
Incompatibilities
An organic carboxylic acid. Keep away from oxidizers, sulfuric acid, caustics, ammonia, aliphatic amines, alkanolamines, isocyanates, alkylene oxides, and epichlorohydrin. Corrosive solution; attacks most common metals. React violently with strong oxidizers, bromine, 90% hydrogen peroxide, phosphorus trichloride, silver powders or dust. Mixture with some silver compounds forms explosive salts of silver oxalate. Incompatible with silver compounds.
Waste Disposal
Recycle any unused portion of the material for its approved use or return it to the manu- facturer or supplier. Ultimate disposal of the chemical must consider: the material’s impact on air quality; potential migration in soil or water; effects on animal, aquatic, and plant life; and conformance with environmental and public health regulations .
Properties of Decanoic acid
| Melting point: | 27-32 °C(lit.) |
| Boiling point: | 268-270 °C(lit.) |
| Density | 0.893 g/mL at 25 °C(lit.) |
| vapor pressure | 15 mm Hg ( 160 °C) |
| refractive index | 1.4169 |
| FEMA | 2364 | DECANOIC ACID |
| Flash point: | >230 °F |
| storage temp. | room temp |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Crystalline Solid |
| pka | 4.79±0.10(Predicted) |
| color | White |
| Odor | Odorless |
| PH | 4 (0.2g/l, H2O, 20℃) |
| Water Solubility | 0.15 g/L (20 º C) |
| Merck | 14,1758 |
| JECFA Number | 105 |
| BRN | 1754556 |
| Stability: | Stable. Incompatible with bases, reducing agents, oxidizing agents. |
| CAS DataBase Reference | 334-48-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Decanoic acid(334-48-5) |
| EPA Substance Registry System | Decanoic acid (334-48-5) |
Safety information for Decanoic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
Computed Descriptors for Decanoic acid
Decanoic acid manufacturer
JSK Chemicals
NRS Chemicals LLP
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Decanoic acid, 98% 98%View Details
Decanoic acid, 98% 98%View Details
334-48-5 -
 334-48-5 Capric acid 98%View Details
334-48-5 Capric acid 98%View Details
334-48-5 -
 Decanoic acid >98% (GC) CAS 334-48-5View Details
Decanoic acid >98% (GC) CAS 334-48-5View Details
334-48-5 -
 Capric AcidView Details
Capric AcidView Details
334-48-5 -
 Capric Acid, Grade Standard: Technical Grade, Packaging Size: 1-2 kgView Details
Capric Acid, Grade Standard: Technical Grade, Packaging Size: 1-2 kgView Details
334-48-5 -
 N-Decanoic Acid CAS: 334-48-5View Details
N-Decanoic Acid CAS: 334-48-5View Details
334-48-5 -
 Capric Acid, Decanoic AcidView Details
Capric Acid, Decanoic AcidView Details
334-48-5 -
 Capric AcidView Details
Capric AcidView Details
334-48-5
