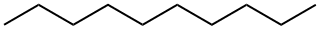1-Nonanol
Synonym(s):1-Nonanol;Alcohol C9;Nonyl alcohol
- CAS NO.:143-08-8
- Empirical Formula: C9H20O
- Molecular Weight: 144.25
- MDL number: MFCD00002990
- EINECS: 205-583-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:59

What is 1-Nonanol?
Description
Nonyl alcohol has a characteristic rose-orange odor and a slightly fatty, bitter taste reminiscent of orange. May be synthesized by reduction of ethyl pelargonate; from heptaldehyde via heptanol and heptylmagnesium bromide with ethylene oxide.
Chemical properties
1-Nonanol is a colourless to light yellow liquid that has a characteristic rose-orange odor and a slightly fatty, bitter taste reminiscent of orange. It can be found in several citrus oils.
Occurrence
Reported as occurring frequently in nature, free or esterified; in the essential oils of grapefruit, Guinea sweet orange, Italian and Israeli sweet orange, bitter orange; and in oak musk concrete. Also reported found in apple, citrus juices, many berries, currants, guava, grapes, papaya, melon, pineapple, asparagus, cucumber, leek, peas, potato, cheeses, butter, milk, cooked chicken, beef and pork, hop oil, beer, rum, grape wines, tea, pecan, peanut oil, soybean, olive, plum, rose apple, beans, mushroom, starfruit, cauliflower, tamarind, fig, cardamom, rice, prickly pear, sweet corn, malt, cherimoya, clary sage, oysters, crab, crayfish, clam, nectarine, mate, pepino fruit (Solanum muricatum), apricot, tobacco, and wheat bread.
The Uses of 1-Nonanol
1-Nonanol is an chain fatty acid alcohol that naturally occurs in oil of orange. 1-Nonanol is used in the manufacture of artificial lemon oil.
The Uses of 1-Nonanol
1-Nonanol is an chain fatty acid alcohol that naturally occurs in oil of orange. 1-Nonanol is used in the manufacture of artificial lemon oil.
Preparation
1-Nonanol can be synthesized by reduction of ethyl pelargonate; from heptaldehyde via heptanol and heptylmagnesium bromide with ethylene oxide.
Production Methods
1-Nonanol is produced by the high-pressure catalytic reduction of esters of pelargonic acid.
Definition
ChEBI: Nonan-1-ol is a fatty alcohol consisting of a hydroxy function at C-1 of an unbranched saturated chain of nine carbon atoms. It has been isolated as a component of volatile oils from plants like Hordeum vulgare. It has a role as a plant metabolite and a volatile oil component. It derives from a hydride of a nonane.
Aroma threshold values
Detection: 50 to 90 ppb; aroma characteristics at 1.0%: intensely waxy, sweet, green, citrus, orange-like, creamy with fruity nuances of tamarind and melon.
Taste threshold values
Taste characteristics at 0.5 to 1.0 ppm: waxy, green, coconut, cheese, milky and creamy with citrus orange nuances.
Synthesis Reference(s)
Tetrahedron Letters, 32, p. 4235, 1991 DOI: 10.1016/S0040-4039(00)92136-1
General Description
Colorless liquid with a rose or fruity odor. Floats on water. Freezing point 23°F.
Reactivity Profile
1-Nonanol is an alcohol. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides.
Health Hazard
Liquid irritates eyes.
Flammability and Explosibility
Not classified
Safety Profile
Mddly toxic by ingestion, skin contact, and inhalation. Experimental reproductive effects. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALCOHOLS.
Metabolism
See alcohol C-8. Nonanol, like other primary alcohols, undergoes two general reactions in vivo. The first is oxidation to the carboxylic acid derivative and next the direct conjugation with glucuronic acid. It was reported that nonanol undergoes direct glucuronic conjugation to the extent of 4.1%. This oxidation proceeds with very little inhibition as opposed to that shown by methyl amyl alcohol and 2-ethyl butyl alcohol which form ester glucuronides.
References
1.https://en.wikipedia.org/wiki/1-Nonanol
2. https://pubchem.ncbi.nlm.nih.gov/compound/1-Nonanol#section=Non-Human-Toxicity-Values
Properties of 1-Nonanol
| Melting point: | -8--6 °C (lit.) |
| Boiling point: | 215 °C (lit.) |
| Density | 0.827 g/mL at 25 °C (lit.) |
| vapor density | 5 (vs air) |
| vapor pressure | 13 mm Hg ( 104 °C) |
| refractive index | n |
| FEMA | 2789 | NONYL ALCOHOL |
| Flash point: | 208 °F |
| storage temp. | Store below +30°C. |
| solubility | 69.54mg/l |
| form | Liquid |
| pka | 15.22±0.10(Predicted) |
| color | Clear colorless |
| Odor | Rose-citrus. |
| Odor Threshold | 0.0009ppm |
| explosive limit | 0.80-6.10%(V) |
| Water Solubility | 1 g/L (20 ºC) |
| Merck | 14,6679 |
| JECFA Number | 100 |
| BRN | 969213 |
| Dielectric constant | 8.8300000000000001 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 143-08-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Nonanol(143-08-8) |
| EPA Substance Registry System | 1-Nonanol (143-08-8) |
Safety information for 1-Nonanol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1-Nonanol
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 143-08-8 NONANOL 98%View Details
143-08-8 NONANOL 98%View Details
143-08-8 -
 1-Nonanol CAS 143-08-8View Details
1-Nonanol CAS 143-08-8View Details
143-08-8 -
 Nonyl alcohol CAS 143-08-8View Details
Nonyl alcohol CAS 143-08-8View Details
143-08-8 -
 1-Nonanol CAS 143-08-8View Details
1-Nonanol CAS 143-08-8View Details
143-08-8 -
 1-Nonanol (Alcohol C9) C9H20O, Industrial GradeView Details
1-Nonanol (Alcohol C9) C9H20O, Industrial GradeView Details
143-08-8 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4