N,N-Dimethylcyclohexylamine
Synonym(s):Cyclohexyldimethylamine;Dimethylaminocyclohexane;N,N-Dimethylcyclohexylamine;N-Cyclohexyldimethylamine
- CAS NO.:98-94-2
- Empirical Formula: C8H17N
- Molecular Weight: 127.23
- MDL number: MFCD00003844
- EINECS: 202-715-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
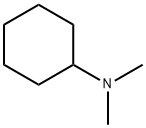
What is N,N-Dimethylcyclohexylamine?
Chemical properties
CLEAR LIQUID
The Uses of N,N-Dimethylcyclohexylamine
Dimethylcyclohexylamine is used in polyurethane plastics and textiles and as a chemical intermediate.
The Uses of N,N-Dimethylcyclohexylamine
N,N-Dimethylcyclohexylamine has been used:
- as switchable hydrophilicity solvent (SHS) for the extraction of lipids from freeze-dried samples of Botryococcus braunii microalgae for biofuel production
- as catalyst in three-component organocatalyzed Strecker reaction on water
Definition
ChEBI: A tertiary amine consisting of cyclohexane having a dimethylamino substituent.
Production Methods
N,N-Dimethylcyclohexylamine is manufactured either by the reaction of methyl chloride or formaldehyde and hydrogen with cyclohexylamine (HSDB 1989).
What are the applications of Application
The curing temperature of baking finishes comprising polyurethane-forming substances can be reduced by 50 – 80 ℃ by adding weakly acidic derivatives of N,N-Dimethylcyclohexylamine. Like pyridine, dimethylcyclohexylamine catalyzes certain reactions and is slightly more efficient than pyridine in the preparation of acid chlorides with thionyl chloride. It can be used as corrosion inhibitor and as an antioxidant in fuel oils.
Synthesis Reference(s)
Journal of the American Chemical Society, 93, p. 2897, 1971 DOI: 10.1021/ja00741a013
Organic Syntheses, Coll. Vol. 6, p. 499, 1988
General Description
Colorless liquid with a musky ammonia odor. Less dense than water.
Air & Water Reactions
Highly flammable. Water soluble.
Reactivity Profile
N,N-Dimethylcyclohexylamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Health Hazard
Inhalation of high concentration of vapor will will produce irritation of the respiratory tract and lungs. Inhalation of large quantities of vapor may be fatal.
Health Hazard
Industrial hygiene studies in polyurethane manufacturing plants have identified levels of 0.007-0.81 p.p.m. N-N-dimethylcyclohexylamine in air; however, these levels were not regarded as hazardous (Reisdorf and Haggerty 1982). There are no current exposure standards for N-N-dimethylcyclohexylamine and no documentation of human toxicological effects.
Fire Hazard
Behavior in Fire: Dangerous when exposed to heat or flame. Can react vigorously with oxidizing materials.
Industrial uses
This amine is used as a catalyst in the production of polyurethane foams. It is also used as an intermediate for rubber accelerators and dyes and in the treatment of textiles.
Safety Profile
Poison by ingestion. Moderately toxic by inhalation. Whenheated to decomposition it emits toxic fumes of NOx
Metabolism
There is no record of any metabolic studies with MTV-dime thy ley clohexylamine. However, one can predict that it would be oxidized to the N-oxide by either a cytochrome P-450 system (Damani 1982) or the flavin-containing monooxygenase (Ziegler 1988). Mixed function oxidase enzymes would be expected to produce demethylation (Lindeke and Cho 1982). Many studies describe the metabolism of the parent compound cyclohexylamine (Henderson 1990).
Properties of N,N-Dimethylcyclohexylamine
| Melting point: | -60 °C |
| Boiling point: | 158-159 °C (lit.) |
| Density | 0.849 g/mL at 25 °C (lit.) |
| vapor pressure | 3.6 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 108 °F |
| storage temp. | Store below +30°C. |
| solubility | 10 g/L (20°C) |
| form | Liquid |
| pka | pK1:10.72(+1) (25°C) |
| color | Clear |
| PH | 12 (5g/l, H2O, 20℃) |
| explosive limit | 3.6-19%(V) |
| Water Solubility | 10 g/L (20 ºC) |
| FreezingPoint | <-77℃ |
| Sensitive | Air Sensitive |
| BRN | 1919922 |
| Dielectric constant | 2.8599999999999999 |
| CAS DataBase Reference | 98-94-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Cyclohexanamine, N,N-dimethyl-(98-94-2) |
| EPA Substance Registry System | N,N-Dimethylcyclohexylamine (98-94-2) |
Safety information for N,N-Dimethylcyclohexylamine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H311:Acute toxicity,dermal H314:Skin corrosion/irritation H330:Acute toxicity,inhalation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for N,N-Dimethylcyclohexylamine
| InChIKey | SVYKKECYCPFKGB-UHFFFAOYSA-N |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran



You may like
-
 N,N-Dimethylcyclohexylamine 99%View Details
N,N-Dimethylcyclohexylamine 99%View Details -
 98-94-2 98%View Details
98-94-2 98%View Details
98-94-2 -
 N,N-Dimethylcyclohexylamine CAS 98-94-2View Details
N,N-Dimethylcyclohexylamine CAS 98-94-2View Details
98-94-2 -
![N,N-Dimethylcyclohexylamine [for HPLC] CAS 98-94-2](https://img.chemicalbook.in//Content/image/CP5.jpg) N,N-Dimethylcyclohexylamine [for HPLC] CAS 98-94-2View Details
N,N-Dimethylcyclohexylamine [for HPLC] CAS 98-94-2View Details
98-94-2 -
 N,N-Dimethylcyclohexylamine CAS 98-94-2View Details
N,N-Dimethylcyclohexylamine CAS 98-94-2View Details
98-94-2 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
