Nickel
Synonym(s):;Nickel;Nickel sponge;Raney Ni
- CAS NO.:7440-02-0
- Empirical Formula: Ni
- Molecular Weight: 58.69
- MDL number: MFCD00011137
- EINECS: 231-111-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
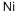
What is Nickel?
Description
Nickel is a hard, silvery white, malleable metal chunk or grey powder. Nickel powder is pyrophoric – can ignite spontaneously. It may react violently with titanium, ammonium nitrate, potassium perchlorate, and hydrazoic acid. It is incompatible with acids, oxidising agents, and sulphur. The industrially important nickel compounds are nickel oxide (NiO), nickel acetate (Ni(C2H3O2), nickel carbonate (NiCO3), nickel carbonyl (Ni(CO)4), nickel subsulphide (NiS2), nickelocene (C5H5)2Ni, and nickel sulphate hexahydrate (NiSO4 · 6H2O).

Nickel compounds have been well established as human carcinogens. Investigations into the molecular mechanisms of nickel carcinogenesis have revealed that not all nickel compounds are equally carcinogenic: certain water-insoluble nickel compounds exhibit potent carcinogenic activity, whereas highly water-soluble nickel compounds exhibit less potency. The reason for the high carcinogenic activity of certain water-insoluble nickel compounds relates to their bioavailability and the ability of the nickel ions to enter cells and reach chromatin. The water-insoluble nickel compounds enter cells quite efficiently via phagocytic processes and subsequent intracellular dissolution. Nickel is classified as a borderline metal ion because it has both soft and hard metal properties and it can bind to sulphur, nitrogen, and oxygen groups. Nickel ions are very similar in structure and coordination properties to magnesium.
Chemical properties
silver white, hard, malleable metal chunks or grey powder
Chemical properties
RANEY NICKEL is a hard, ductile, magnetic metal with a silver-white color.
Physical properties
Nickel metal does not exist freely in nature. Rather, it is located as compounds in ores ofvarying colors, ranging from reddish-brown rocks to greenish and yellowish deposits, andin copper ores. Once refined from its ore, the metallic nickel is a silver-white and hard butmalleable and ductile metal that can be worked hot or cold to fabricate many items. Nickel,located in group 10, and its close neighbor, copper, just to its right in group 11 of the periodictable, have two major differences. Nickel is a poor conductor of electricity, and copper is anexcellent conductor, and although copper is not magnetic, nickel is. Nickel’s melting point is1,455°C, its boiling point is 2,913°C, and its density is 8.912 g/cm3.
Isotopes
There are 31 isotopes of nickel, ranging from Ni-48 to Ni-78. Five of these arestable, and the percentage of their contribution to the element’s natural existence onEarth are as follows: Ni-58 = 68.077%, Ni-60 = 26.223%, Ni-61 = 1.140%, Ni-62 =3.634%, and Ni 64 = 0.926%. All of the other 26 isotopes of nickel are artificially madeand radioactive with half-lives ranging from a few nanoseconds to 7.6×104 years.
Origin of Name
The name is derived from the ore niccolite, meaning “Old Nick,” referred to as the devil by German miners. The niccolite mineral ore was also called “kupfernickel,” which in German stands for two things; first, it is the name of a gnome (similar to Cobalt), and second, it refers to “Old Nick’s false copper.”
Occurrence
Nickel is the 23rd most abundant element found in the Earth’s crust. It is somewhat plentiful but scattered and makes up one-hundredth of 1% of igneous rocks. Nickel metal is foundin meteorites (as are some other elements). It is believed that molten nickel, along with iron,makes up the central sphere that forms the core of the Earth.There are several types of nickel ores. One is the major ore for nickel called pentlandite(NiS ? 2FeS), which is iron/nickel sulfide. Another is a mineral called niccolite (NiAs), discovered in 1751 and first found in a mining area of Sweden. By far, the largest mining area fornickel is located in Ontario, Canada, where it is recovered from what is thought to be a verylarge meteorite that crashed into the Earth eons ago. This large nickel deposit is one reasonfor the theory of the Earth’s core being molten nickel and iron, given that both the Earth andmeteorites were formed during the early stages of the solar system. Some nickel ores are alsofound in Cuba, the Dominican Republic, and Scandinavia. Traces of nickel exist in soils, coal,plants, and animals.
History
Discovered by Cronstedt in 1751 in kupfernickel (niccolite). Nickel is found as a constituent in most meteorites and often serves as one of the criteria for distinguishing a meteorite from other minerals. Iron meteorites, or siderites, may contain iron alloyed with from 5 to nearly 20% nickel. Nickel is obtained commercially from pentlandite and pyrrhotite of the Sudbury region of Ontario, a district that produces much of the world’s nickel. It is now thought that the Sudbury deposit is the result of an ancient meteorite impact. Large deposits of nickel, cobalt, and copper have recently been developed at Voisey’s Bay, Labrador. Other deposits of nickel are found in Russia, New Caledonia, Australia, Cuba, Indonesia, and elsewhere. Nickel is silvery white and takes on a high polish. It is hard, malleable, ductile, somewhat ferromagnetic, and a fair conductor of heat and electricity. It belongs to the iron-cobalt group of metals and is chiefly valuable for the alloys it forms. It is extensively used for making stainless steel and other corrosion- resistant alloys such as Invar?, Monel?, Inconel?, and the Hastelloys?. Tubing made of a copper-nickel alloy is extensively used in making desalination plants for converting sea water into fresh water. Nickel is also now used extensively in coinage and in making nickel steel for armor plate and burglar-proof vaults, and is a component in Nichrome?, Permalloy?, and constantan. Nickel added to glass gives a green color. Nickel plating is often used to provide a protective coating for other metals, and finely divided nickel is a catalyst for hydrogenating vegetable oils. It is also used in ceramics, in the manufacture of Alnico magnets, and in batteries. The sulfate and the oxides are important compounds. Natural nickel is a mixture of five stable isotopes; twenty-five other unstable isotopes are known. Nickel sulfide fume and dust, as well as other nickel compounds, are carcinogens. Nickel metal (99.9%) is priced at about $2/g or less in larger quantities.
Characteristics
As mentioned, nickel is located in group 10 (VIII) and is the third element in the specialtriad (Fe, Co, Ni) of the first series of the transition elements. Nickel’s chemical and physicalproperties, particularly its magnetic peculiarity, are similar to iron and cobalt.Some acids will attack nickel, but it offers excellent protection from corrosion from air andseawater. This quality makes it excellent for electroplating other metals to form a protectivecoating. Nickel is also an excellent alloy metal, particularly with iron, for making stainless steelas well as a protective armor for military vehicles. It is malleable and can be drawn throughdies to form wires. About one pound of nickel metal can be drawn to about 200 miles of thinwire.
The Uses of Nickel
Nickel-plating; for various alloys such as new silver, Chinese silver, German silver; for coins, electrotypes, storage batteries; magnets, lightning-rod tips, electrical contacts and electrodes, spark plugs, machinery parts; catalyst for hydrogenation of oils and other organic substances. See also Raney nickel. manufacture of Monel metal, stainless steels, heat resistant steels, heat and corrosion resistant alloys, nickel-chrome resistance wire; in alloys for electronic and space applications.
The Uses of Nickel

To a shaker flask was added the SM (crude, 32.4 g, 138 mmol), EtOH (100 mL), and Raney Ni (1.00 g, 17.04 mmol). The flask was charged with H2 (275 kPa) and was agitated until the absorption of H2 ceased. The vessel was depressurized and the catalyst was removed via filtration. The filtrate was concentrated to dryness, diluted with MTBE, and filtered again. The filtrate was concentrated and the resulting residue was stirred in hexane. The solids were filtered, washed with cold hexane, and dried in vacuo to provide the product as a dark solid. [17.8 g, 63%]
The Uses of Nickel
Nickel is used in various alloys, such asGerman silver, Monel, and nickel–chrome;for coins; in storage batteries; in spark plugs;and as a hydrogenation catalyst.
The Uses of Nickel
The most common use of nickel is as an alloy metal with iron and steel to make stainlesssteel, which contains from 5% to 15% nickel. The higher the percentage of nickel in stainlesssteel, the greater the steel’s resistance to corrosion—particularly when exposed to seawater.Nickel is also alloyed with copper to make Monel metal, which was widely used before stainless steel became more economical and practical. It was used for many purposes as varied ashousehold appliances and general manufacturing. Nickel is also used to electroplate othermetals to provide a noncorrosive protective and attractive finish.
Definition
ChEBI: Chemical element (nickel group element atom) with atomic number 28.
Definition
A transition metal that occurs naturally as the sulfide and silicate. It is extracted by the Mond process, which involves reduction of nickel oxide using carbon monoxide followed by the formation and subsequent decomposition of volatile nickel carbonyl. Nickel is used as a catalyst in the hydrogenation of alkenes, e.g. margarine manufacture, and in coinage alloys. Its main oxidation state is +2 and these compounds are usually green. Symbol: Ni; m.p. 1453°C; b.p. 2732°C; r.d. 8.902 (25°C); p.n. 28; r.a.m. 58.6934.
Definition
nickel: Symbol Ni. A malleable ductilesilvery metallic transition element;a.n. 28; r.a.m. 58.70; r.d. 8.9;m.p. 1450°C; b.p. 2732°C. It is foundin the minerals pentlandite (NiS),pyrrhoite ((Fe,Ni)S), and garnierite((Ni,Mg)6(OH)6Si4O11.H2O). Nickel isalso present in certain iron meteorites(up to 20%). The metal isextracted by roasting the ore to givethe oxide, followed by reductionwith carbon monoxide and purificationby the Mond process. Alternativelyelectolysis is used. Nickel metalis used in special steels, in Invar, and,being ferromagnetic, in magnetic alloys,such as Mumetal. It is also aneffective catalyst, particularly for hydrogenation reactions (see also raneynickel). The main compounds areformed with nickel in the +2 oxidationstate; the +3 state also exists (e.g.the black oxide, Ni2O3). Nickel wasdiscovered by Axel Cronstedt(1722–65) in 1751.
Production Methods
Nickel is obtained by processing sulfide and laterite ore
concentrates using pyrometallurgic and hydrometallurgic
processes. The resultant nickel matte obtained by roasting
and smelting is subjected to further cleaning by electro-,
vapo-, and hydrometallurgic refining methods. Some portion
of the matte is roasted to obtain commercial nickel oxide
agglomerate. Pure, 99.9% nickel can be obtained by electrolytic
refining process.
The most pure, 99.97%, nickel is obtained by vapometallurgy.
In this process, known also as the Mond method,nickel and copper sulfide blend is converted to oxides and
then reduced by heating with water gas at 350–400°C. The
resultant active form of nickel is treated with carbon monoxide
to give volatile nickel carbonyl [Ni(CO)4]. The latter
reaction is reversible; heating results in pure nickel and
carbon monoxide.
Preparation
The carbonyl process is most commonly employed when very pure nickel is required.
The impure metal is reacted with pure carbon monoxide at 50° and the carbonyl produced
fractionated several times prior to pyrolysis at around 200°. The nickel thus obtained
has a purity of 99.90-99.99% depending upon the materials used.
Electrolytic methods for producing high purity nickel depend upon the production of high purity nickel salts. The nickel obtained by the electrolysis of pure nickel chloride solution with inert platinum-iridium anodes is 99.99% pure.
General Description
Nickel catalyst, is extremely fine powdered nickel. Nickel is grayish colored. Insoluble in water. Nickel catalyst is used to promote the chemical action in manufacturing synthetics and to process vegetable oil and petroleum. If exposed to air or moisture, Nickel may become hot enough to ignite. Nickel is insoluble in water and does not react with larger volumes of water.
Air & Water Reactions
Pyrophoric, Ignites spontaneously in the presence of air; during storage, H2 escapes with fire and explosion hazards; reacts violently with acids forming H2. [Handling Chemicals Safely 1980. p. 807].
Reactivity Profile
Metals, such as METAL CATALYST, are reducing agents and tend to react with oxidizing agents. Their reactivity is strongly influenced by their state of subdivision: in bulk they often resist chemical combination; in powdered form they may react very rapidly. Thus, as a bulk metal Nickel is somewhat unreactive, but finely divided material may be pyrophoric. The metal reacts exothermically with compounds having active hydrogen atoms (such as acids and water) to form flammable hydrogen gas and caustic products. The reactions are less vigorous than the similar reactions of alkali metals, but the released heat can still ignite the released hydrogen. Materials in this group may react with azo/diazo compounds to form explosive products. These metals and the products of their corrosion by air and water can catalyze polymerization reactions in several classes of organic compounds; these polymerizations sometimes proceed rapidly or even explosively. Some metals in this group form explosive products with halogenated hydrocarbons. Can react explosively with oxidizing materials.
Hazard
Nickel dust and powder are flammable. Most nickel compounds, particularly the salts, aretoxic. NiSO4 is a known carcinogen.
Although nickel is not easily absorbed in the digestive system, it can cause toxic reactionsand is a confirmed carcinogen in high concentration in the body. Nickel workers can receivesevere skin rashes and lung cancer from exposure to nickel dust and vapors.
Nickel is stored in the brain, spinal cord, lungs, and heart. It can cause coughs, shortnessof breath, dizziness, nausea, vomiting, and general weakness.
Health Hazard
Ingestion of nickel can cause hyperglycemia,depression of the central nervous system,myocardial weakness, and kidney damage.A subcutaneous lethal dose in rabbits isin the range 10 mg/kg. The oral toxicityof the metal, however, is very low. Skincontact can lead to dermatitis and “nickelitch,” a chronic eczema, caused by dermalhypersensitivity reactions. Nickel itch mayresult from wearing pierced earrings. Inhalationof metal dusts can produce irritation ofthe nose and respiratory tract. Nickel andsome of its compounds have been reportedto cause lung cancer in experimental animals.It may also induce cancer in nose,stomach, and possibly the kidney. The experimentaldata on the latter, are not fully confirmative.Nickel refinery flue dust, nickelsulfide (Ni3S2) , and nickeloxide (NiO) produced localizedtumors in experimental animals wheninjected intramuscularly. IARC has classifiednickel and its compounds as carcinogenicto humans (IARC 1990). Inhalation ofmetal dusts can produce lung and sinus cancersin humans, with a latent period of about25 years.
Nickel is susceptible to cross human placentaand produce teratogenesis and embroytoxicity. In vitro study on lipid peroxidationindicated that nickel induced peroxidativedamage to placental membrane causing decreased placental viability, altered permeabilityand subsequent embroy toxicity (Chenand Lin 1998). In a latter study, Chen et al.(2003) evaluated nickel-induced oxidativestress and effects of antioxidants in humanlymphocytes. The levels of intracellular reactiveoxygen species, lipid peroxidation andhydroxyl radicals were examined for one hourfollowing acute treatment with Nicl2. Thestudy showed that glutathione, catalase andmannitol each provided protection against theoxidative stress induced by Ni.
The efficacy of organic chelating ligandsin cleaning human skin contaminated withnickel has been investigated (Healy et al.1998). Commercial liquid soap added withL-histidine was found to be more effectivethan the untreated soap. Similarly sodiumethylenediamine tetraacetic acid (EDTA)salt or L-histidine added to phosphate buffersaline solution was more effective in cleaningnickel contaminated human skin than thephosphate saline alone.
Fire Hazard
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.
Agricultural Uses
Nickel (Ni) is a silver-white, ductile, malleable, yet
tough metallic element of Group 10 (formerly Group
VIII) of the Periodic Table. Mostly, nickel
goes into the making of steel and other corrosion resistant
alloys. Finely divided nickel is used as a hydrogenation
catalyst. Nickel is a beneficial trace element for plants.
Its presence in the urease enzyme underlines its
importance as a functional element. It is essential for
grain viability, in barley and at concentrations less than
100 μg/kg, the grain level and the germination frequency
decrease progressively. The quantity of Ni in a few
fertilizers is as given: 2 ppm in nitrochalk, 13 ppm in
superphosphate and 10 ppm in FYM.
Nickel is the metal component of urease that
hydrolyzes urea to give ammonia and carbon dioxide.
Compounds that react with nickel in the urease molecule
inhibit the hydrolysis of urea.
Nickel enhances the nodule weight and the seed yield
of soybeans, chickpeas and temperate cereals. It is
present in plants in the range of 0.1 to 1O ppm of the dry
weight.
High levels of Ni may induce Zn or Fe deficiency
because of cation competition, and may create nickel
toxicity. The browning and necrosis of the leaf tips and
margins are the toxicity symptoms on the plant. High Ni
content also causes the distortion of young leaves and the
death of the terminal shoots of the plant. The emerging
leaves may fail to unroll and become necrotic, with the
necrosis starting from near the base and spreading toward
the leaf tip. Nickel toxicity in cereals and grasses varies
in the intensity of chlorosis along the length of the leaf
with a series of transverse bands.
Sewage sludge contains heavy metals like Ni, Cd, etc.
that are absorbed by plants grown in soils contaminated
with these heavy metals. The toxicity caused by these
metals is in turn, passed on to animals that feed on such
plants. Any regulation for sludge use should ensure that
the soil pH is not lower than 6.5, as heavy metals are
insoluble at pH greater than 6.5.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. Poison by ingestion, intratracheal, intraperitoneal, subcutaneous, and intravenous routes. An experimental teratogen. Ingestion of soluble salts causes nausea, vomiting, and diarrhea. Mutation data reported. Hypersensitivity to nickel is common and can cause allergic contact dermatitis, pulmonary asthma, conjunctivitis, and inflammatory reactions around nickel-containing medcal implants and prostheses. Powders may ignite spontaneously in air. Reacts violently with F2, NH4NO3, hydrazine, NH3, (H2 + dioxane), performic acid, P, Se, S, (Ti + KCLO3). Incompatible with oxidants (e.g., bromine pentafluoride, peroxyformic acid, potassium perchlorate, chlorine, nitryl fluoride, ammonium nitrate), Raney-nickel catalysts may initiate hazardous reactions with ethylene + aluminum chloride, pdioxane, hydrogen, hydrogen + oxygen, magnesium silicate, methanol, organic solvents + heat, sulfur compounds. Nickel catalysts have caused many industrial accidents.
Potential Exposure
Nickel is used as an alloy additive in steel manufacture; in the production of coins and other utensils. Nickel forms alloys with copper, manganese, zinc, chromium, iron, molybdenum, etc. Stainless steel is the most widely used nickel alloy. An important nickel copper alloy is Monel metal, which contains 66% nickel and 32% copper and has excellent corrosion resistance properties. Permanent magnets are alloys chiefly of nickel, cobalt, aluminum, and iron. Elemental nickel is used in electroplating, anodizing aluminum casting operations for machine parts; and in coinage; in the manufacture of acid-resisting and magnetic alloys; magnetic tapes; surgical and dental instruments; nickel cadmium batteries; nickel soaps in crankcase oil; in ground-coat enamels; colored ceramics; and glass. It is used as a catalyst in the hydrogenation synthesis of acrylic esters for plastics. Exposure to nickel may also occur during mining, smelting, and refining operations. The route by which most people in the general population receive the largest portion of daily nickel intake is through food. Based on the available data from composite diet analysis, between 300 and 600 μg nickel per day are ingested. Fecal nickel analysis, a more accurate measure of dietary nickel intake, suggests about 300 μg per day. The highest level of nickel observed in water was 75 μg/L. Average drinking water levels are about 5 μg/L. A typical consumption of 2 L daily would yield an additional 10 μg of nickel, of which up to 1 μg would be absorbed.
Carcinogenicity
Metallic nickel is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
Nickel and its compounds are naturally present in the Earth’s
crust, and nickel can be released into the atmosphere via
natural discharges such as windblown dust and volcanic
eruptions. It is estimated that 8.5 million kilograms of nickel
are emitted into the atmosphere from natural sources such
as windblown dust, volcanoes, and vegetation each year.
Anthropogenic activities constitute significant discharge into
the environment, particularly in the form of particulate matter
and nickel compounds not normally found naturally; these
sources comprise five times the quantity estimated to come
from natural sources.
Nickel releases are mainly in the form of aerosols that cover
a broad spectrum of sizes. Particulates from power plants tend
to be associated with smaller particles than those from
smelters. Atmospheric aerosols are removed by gravitational
settling and dry and wet deposition. Submicrometer particles
may have atmospheric half-lives as long as 30 days. Monitoring
data confirm that nickel can be transported far from its source,
and that the form of nickel emitted to the atmosphere will vary
according to the type of source. Species associated with
combustion, incineration, and metals smelting and refining are
often complex nickel oxides, nickel sulfate, metallic nickel, and
in more specialized industries, nickel silicate, nickel subsulfide,
and nickel chloride.
Nickel may be transported into streams and waterways from
the natural weathering of soil as well as from anthropogenic
discharges and runoff. This nickel can accumulate in sediment,
with the adsorption of the metal to the soil depending on pH,
redox potential, ionic strength of the water, concentration of
complexing ions, and the metal concentration and type.
Soluble nickel compounds such as nickel chloride would be
expected to release divalent nickel into moist environments.
Since these compounds quickly dissolve upon exposure to
water, and partially due to the ubiquity of nickel in soil, water,
and air, tracking the course of these compounds through the
environment is difficult. This is particularly due to nickel’s
ability to complex with anionic species other than chloride to
form nickel oxide, sulfate, nitrate, carbonate, or acetate, among
others.
Industrial uses of nickel result in nickel being distributed
mainly at soil surfaces and through surrounding waterways and
water tables. Once distributed to the soil, nickel(II) ions can
potentially form inorganic crystalline minerals or precipitates,
can complex or adsorb onto organic and inorganic surfaces, can
participate in cation exchange, and can exist as free-ion or
chelated metal complexes in soil solution.
Shipping
UN3089 Metal powders, flammable, n.o.s., Hazard Class: 4.1; Labels: 4.1-Flammable solid. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard Class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Toxicity evaluation
Skin sensitization is believed to occur as a result of nickel
binding to proteins (particularly on the cell surface) and
hapten formation. The nickel–protein complex is recognized as
foreign and an immune reaction follows. For example, sweat
may react with the nickel in plated jewelry that comes in direct
contact with skin; dissolved metal may penetrate and react with
proteins in the skin, leading to immune sensitization. Nickel
may substitute for certain other metals (especially zinc) in
metal-dependent enzymes, leading to altered protein function.
High nickel content in serum and tissue may interfere with
both copper and zinc metabolism. It also readily crosses the cell
membrane via calcium channels and competes with calcium
for specific receptors.
Nickel can alter the sodium balance and lipid metabolism
and can induce metallothionein synthesis. Dissolved nickel
also affects the T-cell system and suppresses the activity of
natural killer cells. If given orally or by inhalation, nickel
chloride has been reported to decrease iodine uptake by the
thyroid gland. The lipid peroxidation properties of nickel can
introduce potential malignancies in humans, as DNA strand
gaps and breaks in DNA–protein cross-links can form. The
down-regulation of glycoprotein metabolism by nickel ions
may produce nephrotoxicity in humans as well. Nickel
carbonyl can cross-link amino acids to DNA and lead to
formation of reactive oxygen species. Nickel carbonyl can also
suppress natural killer cell activity and production of some
interferons.
Responses in many of these assays were weak and occurred
at toxic doses, and were affected by tissue culture conditions
modifying uptake by the cell. The mechanism of nickel carcinogenesis
is controversial, and is likely to vary with the form of
nickel. The nickel ion (Ni2+) alone does not form premutagenic
DNA lesions, suggesting that nickel causes indirect DNA
damage, perhaps due to oxidative stress or blocking DNA repair
mechanisms.
Nickel is an essential trace nutrient in plants and certain
animal species (e.g., rat and chick); however, it has not been
shown to be essential in humans.
Incompatibilities
Nickel dust is a spontaneously flammable solid and a dangerous fire hazard.
Waste Disposal
Nickel compoundsencapsulation followed by disposal in a chemical waste landfill. However, nickel from various industrial wastes may also be recovered and recycled as described in the literature.
Properties of Nickel
| Melting point: | 1453 °C (lit.) |
| Boiling point: | 2732 °C (lit.) |
| Density | 8.9 g/mL at 25 °C (lit.) |
| vapor density | 5.8 (vs air) |
| storage temp. | no restrictions. |
| solubility | insoluble in H2O; slightly soluble in dilute acid solutions |
| form | wire |
| appearance | Grey powder |
| color | White to gray-white |
| Specific Gravity | 8.9 |
| PH Range | 9 - 11 at 20 °C |
| Odor | Odorless |
| PH | 8.5-12.0 |
| Resistivity | 6.97 μΩ-cm, 20°C |
| Water Solubility | It is insoluble in water. |
| Sensitive | air sensitive |
| Merck | 14,8107 |
| Exposure limits | TLA-TWA (metal) 1 mg/m3 (ACGIH,
MSHA, and OSHA); (soluble inorganic compounds)
0.1 mg(Ni)/m3 (ACGIH) 0.015 mg
(Ni)/m3 (NIOSH); (insoluble inorganic compounds)
1 mg/m3 (ACGIH). |
| Stability: | Stable in massive form. Powder is pyrophoric - can ignite spontaneously. May react violently with titanium, ammonium nitrate, potassium perchlorate, hydrazoic acid. Incompatible with acids, oxidizing agents, sulfur. |
| CAS DataBase Reference | 7440-02-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Nickel(7440-02-0) |
| IARC | 2B (Vol. Sup 7, 49) 1990 |
| EPA Substance Registry System | Nickel (7440-02-0) |
Safety information for Nickel
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Nickel
| InChIKey | PXHVJJICTQNCMI-UHFFFAOYSA-N |
Nickel manufacturer
JSK Chemicals
Metal Chem India
Gorwara Chemical Industries
Bondbay Pharmaceuticals Pvt Ltd
ARRAKIS INDUSTRIES LLP
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 Raney Nickel 99%View Details
Raney Nickel 99%View Details -
 Raney Nickel 99%View Details
Raney Nickel 99%View Details -
 Nickel CAS 7440-02-0View Details
Nickel CAS 7440-02-0View Details
7440-02-0 -
 Nickel CAS 7440-02-0View Details
Nickel CAS 7440-02-0View Details
7440-02-0 -
 Nickel CAS 7440-02-0View Details
Nickel CAS 7440-02-0View Details
7440-02-0 -
 Nickel wire, 0.5mm (0.02 in.) dia, annealed CAS 7440-02-0View Details
Nickel wire, 0.5mm (0.02 in.) dia, annealed CAS 7440-02-0View Details
7440-02-0 -
 Nickel CAS 7440-02-0View Details
Nickel CAS 7440-02-0View Details
7440-02-0 -
 Nickel CASView Details
Nickel CASView Details