Tosylmethyl isocyanide
Synonym(s):(p-Tolylsulfonyl)methyl isocyanide;TosMIC;Tosylmethyl isocyanide
- CAS NO.:36635-61-7
- Empirical Formula: C9H9NO2S
- Molecular Weight: 195.24
- MDL number: MFCD00000005
- EINECS: 253-140-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
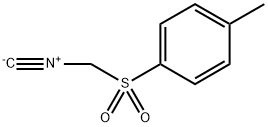
What is Tosylmethyl isocyanide?
Description
Tosylmethyl isocyanide is a versatile synthon in organic chemistry, widely used as a reagent for the preparation of biologically active pyrroles and imidazoles. Tosylmethyl isocyanide undergoes a base-promoted 1,3-dipolar cycloaddition reaction with immobilised imines under microwave irradiation to give 1,5-disubstituted imidazoles.
Chemical properties
Pale yellow to light brown crystalline powder
The Uses of Tosylmethyl isocyanide
Tosylmethyl Isocyanide is used as a synthetic reagent in the preparation of variety or biologically active heterocycles such as pyrroles and imidazoles. Tosylmethyl Isocyanide is reported to inhibit [ Fe]-hydrogenase with very high affinity.
Preparation
N-(p-Tolylsulfonylmethyl)formamide 1609:
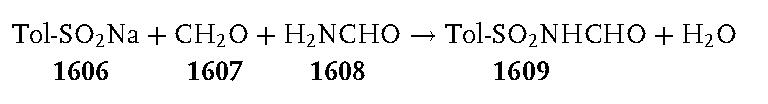
A 3-L, three-necked, round-bottomed flask, equipped with a mechanical stirrer, a condenser, and a thermometer, was charged with sodium p-toluenesulfinate 1606 (267 g, 1.5 mol). After the addition of water (750 mL), a 34–37% solution of formaldehyde 1607 (350 mL, 378 g, ca. 4.4 mol), formamide 1608 (600 mL, 680 g, 15 mol), and formic acid (200 mL, 244 g, 5.3 mol), the stirred reaction mixture was heated at 90 C°. The sodium p-toluenesulfinate dissolved during the heating, and the clear solution was kept at 90–95 C° for 2 h. It was then cooled in an ice/salt bath with continued stirring and further cooled overnight in a freezer at 20 C°. The white solid produced was collected by suction filtration. It was washed thoroughly in a beaker by stirring with three 250 mL portions of iced water. The product was dried under reduced pressure over phosphorus pentoxide at 70 C° to provide 134–150 g (42–47%) of crude N-(p-tolylsulfonylmethyl)formamide 1609; mp 106–110 C°. This product was sufficiently pure to be used directly in the following reaction.
Definition
Tosylmethyl isocyanide is a chemical compound of cyanide that Versatile synthon in organic chemistry, especially in the synthesis of heterocyclic Compounds.
What are the applications of Application
Tosylmethyl isocyanide was used:
(1) in the synthesis of triplet drugs with the 1,3,5-trioxazatriquinane skeleton;
(2) as reagent in the preparation of biologically active pyrroles and imidazoles;
(3) as the isonitrile component in a diastereoselective Passerini reaction employing sugar-derived aldehydes.
Purification Methods
Use an efficient fume cupboard. Purify TOSMIC by dissolving (50g) in CH2Cl2 (150mL) and passing it through a column (40x3cm) containing neutral alumina (100g) in CH2Cl2 and eluting with CH2Cl2. A nearly colourless solution (700mL) is collected, evaporated in vacuo and the residue (42-47g) of TOSMIC (m 113-114o dec) is recrystallised once from MeOH (m 116-117o dec). [Hoogenboom et al. Org Synth 57 102 1977, Lensen Tetrahedron Lett 2367 1972.] It also crystallises from EtOH (charcoal) [Saito & Itano, J Chem Soc, Perkin Trans 1 1 1986].
Hazard
Tosylmethyl isocyanide is toxic to humans and may cause irritation on contact; it is toxic by ingestion, inhalation and skin absorption. Short-term exposure to high concentrations of cyanide can damage the brain and heart and can even lead to coma, convulsions, apnoea, cardiac arrest and death. Prolonged inhalation of cyanide can cause breathing difficulties, chest pain, vomiting, blood changes, headaches and goiter. Skin contact with cyanide salts can cause irritation and ulcers.
Toxicity
Cyanide is an inhibitor of cytochrome c oxidase in the fourth complex of the electron transport chain (found in the membrane of the mitochondria of eukaryotic cells). It complexes with the ferric iron atom in this enzyme. The binding of cyanide to this cytochrome prevents transport of electrons from cytochrome c oxidase to oxygen. As a result, the electron transport chain is disrupted and the cell can no longer aerobically produce ATP for energy. Tissues that mainly depend on aerobic respiration, such as the central nervous system and the heart, are particularly affected. Cyanide is also known produce some of its toxic effects by binding to catalase, glutathione peroxidase, methemoglobin, hydroxocobalamin, phosphatase, tyrosinase, ascorbic acid oxidase, xanthine oxidase, succinic dehydrogenase, and Cu/Zn superoxide dismutase. Cyanide binds to the ferric ion of methemoglobin to form inactive cyanmethemoglobin.
Properties of Tosylmethyl isocyanide
| Melting point: | 109-113 °C(lit.) |
| Density | 1.2721 (rough estimate) |
| refractive index | 1.5270 (estimate) |
| storage temp. | 2-8°C |
| solubility | water: slightly soluble |
| form | Liquid |
| color | Clear |
| Water Solubility | insoluble |
| Sensitive | Moisture Sensitive |
| Merck | 14,9556 |
| BRN | 3592382 |
| Exposure limits | NIOSH: IDLH 25 mg/m3 |
| CAS DataBase Reference | 36635-61-7(CAS DataBase Reference) |
Safety information for Tosylmethyl isocyanide
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Tosylmethyl isocyanide
| InChIKey | BBNNLJMGPASZPD-UHFFFAOYSA-N |
Tosylmethyl isocyanide manufacturer
KP INNOVATIVE
SRINI PHARMACEUTICALS PVT LTD
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 36635-61-7 p-Toluenesulfonylmethyl isocyanide 99%View Details
36635-61-7 p-Toluenesulfonylmethyl isocyanide 99%View Details
36635-61-7 -
 36635-61-7 P-toluenesulfonylmet hyliscyanide 98%View Details
36635-61-7 P-toluenesulfonylmet hyliscyanide 98%View Details
36635-61-7 -
 p-Toluenesulfonylmethyl isocyanide, 98% CAS 36635-61-7View Details
p-Toluenesulfonylmethyl isocyanide, 98% CAS 36635-61-7View Details
36635-61-7 -
 p-Toluenesulfonylmethyl Isocyanide CAS 36635-61-7View Details
p-Toluenesulfonylmethyl Isocyanide CAS 36635-61-7View Details
36635-61-7 -
 Tosylmethyl isocyanide 95% CAS 36635-61-7View Details
Tosylmethyl isocyanide 95% CAS 36635-61-7View Details
36635-61-7 -
 Tosylmethyl isocyanide 97% (HPLC) CAS 36635-61-7View Details
Tosylmethyl isocyanide 97% (HPLC) CAS 36635-61-7View Details
36635-61-7 -
 p-Toluenesulfonylmethyl isocyanide CAS 36635-61-7View Details
p-Toluenesulfonylmethyl isocyanide CAS 36635-61-7View Details
36635-61-7 -
 1- Isocyanomethanesulfonyl-4-methylbenzeneView Details
1- Isocyanomethanesulfonyl-4-methylbenzeneView Details
36635-61-7
