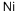CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Lustrous, silvery, odorless metallic solid. Insoluble in water. |
|---|---|
| Boiling Point | 5139 °F at 760 mmHg (NIOSH, 2023) |
| Melting Point | 2831 °F (NIOSH, 2023) |
| Solubility | Insoluble (NIOSH, 2023) |
| Density | 8.9 (Metal) (NIOSH, 2023) - Denser than water; will sink |
| Vapor Pressure | 0 mmHg (approx) (NIOSH, 2023) |
| Chemical Classes | Metals -> Nickel Compounds, Inorganic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 58.693 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 57.935342 g/mol |
| Monoisotopic Mass | 57.935342 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 1 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Nickel is a very abundant natural element. Pure nickel is a hard, silvery-white metal. Nickel can be combined with other metals, such as iron, copper, chromium, and zinc, to form alloys. These alloys are used to make coins, jewelry, and items such as valves and heat exchangers. Most nickel is used to make stainless steel. Nickel can combine with other elements such as chlorine, sulfur, and oxygen to form nickel compounds. Many nickel compounds dissolve fairly easy in water and have a green color. Nickel compounds are used for nickel plating, to color ceramics, to make some batteries, and as substances known as catalysts that increase the rate of chemical reactions. Nickel is found in all soil and is emitted from volcanoes. Nickel is also found in meteorites and on the ocean floor. Nickel and its compounds have no characteristic odor or taste.