Cupric oxide
Synonym(s):Copper monoxide granulated;Copper(II) oxide;Cupric oxide
- CAS NO.:1317-38-0
- Empirical Formula: CuO
- Molecular Weight: 79.55
- MDL number: MFCD00010979
- EINECS: 215-269-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:59
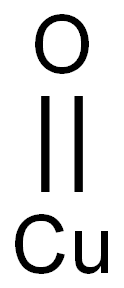
What is Cupric oxide?
Absorption
Following oral administration, copper is mainly absorbed through the gastrointestinal tract from the stomach, duodenum, and jejunum. All other intakes of copper (inhalation and dermal) are insignificant in comparison to the oral route. The bioavailability of copper from cupric oxide depends on the solubilization of the oxide in the gastrointestinal tract . According to studies on cattle and swine, copper oxide displays low absorption rate and high excretion rate . In rats exposed to aerosols containing 50-80 mg/m^3, pulmonary uptake of copper oxide occurred .
Toxicity
Copper toxicity involves gastrointestinal irritation and liver and kidney toxicity. Reported No-Observed-Adverse-Effect-Levels (NOAELs) of copper are in the range of 23-104 mg/kg bw/day, but kidney effects have been shown in male rats at levels as low as 10 mg/kg bw/day . Severe intoxication is associated with serum copper levels greater than 500 mcg/dL. The estimated lethal dose in an untreated adult is 10 to 20 g copper .
Description
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds.Mainly used in wood preservatives, ceramics, and mineral supplements for animal feed.
Copper(II) oxide nanoparticles (NPCuO) have industrial applications as antimicrobial agents in textiles and paints, and catalysts in organic synthesis. They may also be produced from electronic wastes. Cupric oxide poses potential health and environmental concern due to toxic and mutagenic particles generating reactive oxygen species.
Chemical properties
Black fine free powder
Chemical properties
Copper metal, metal compounds and alloys are often used in “hot” operations in the workplace. The workplace operations include, but are not limited to, welding, brazing, soldering, plating, cutting, and metalizing. At the high temperatures reached in these operations, metals often form metal fumes that have different health effects.
The Uses of Cupric oxide
Cupric Oxide can be used as a dietary ingredient and as a nutrient. Copper aids in the absorption of iron, in the formation of red blood cells and the proper bone formation and maintenance.
As pigment in glass, ceramics, enamels, porcelain glazes, artificial gems; in manufacture of rayon, other Cu Compounds; in sweetening petroleum gases; in galvanic electrodes; as flux in metallurgy; in correcting Cu deficiencies in soil; as optical-glass polishing agent; in antifouling paints, pyrotechnic compositions; as exciter in phosphor mixtures; as catalyst for organic reactions; in high tempereture superconductors.
The Uses of Cupric oxide
- Nanoscale Copper(II) Oxide has been studied as photocatalysts, sensors, lubricant additives and batteries.
- Nanorods of cupric oxide have also shown advantages as oxidizing agents in high speed chemical reactions over traditional cupric oxide nanoparticles.
- Copper(II) oxide is a promising p-type oxide material although with a small band gap.
Background
Cupric oxide, or copper (II) oxide, is an inorganic compound with the chemical formula CuO. Cupric oxide is used as a precursor in many copper-containing products such as wood preservatives and ceramics. Cupric oxide may be found in over-the-counter vitamin-mineral supplements as a source of Copper. The mean daily dietary intake of copper in adults ranges between 0.9 and 2.2 mg . Common routes of cupric oxide exposure include ingestion, dermal exposure and inhalation. Copper(II) oxide nanoparticles (NPCuO) have industrial applications as antimicrobial agents in textiles and paints, and catalysts in organic synthesis . They may also be produced from electronic wastes. Cupric oxide poses potential health and environmental concern due to toxic and mutagenic particles generating reactive oxygen species .
Indications
No FDA- or EMA-approved therapeutic indications.
What are the applications of Application
Copper II Oxide is a reagent used as a pigment in ceramics
Definition
A black solid prepared by the action of heat on copper(II) nitrate, hydroxide, or carbonate. It is a basic oxide and reacts with dilute acids to form solutions of copper(II) salts. Copper(II) oxide can be reduced to copper by heating in a stream of hydrogen or carbon monoxide. It can also be reduced by mixing with carbon and heating the mixture. Copper(II) oxide is stable up to its melting point, after which it decomposes to give oxygen, copper(I) oxide, and eventually copper.
Reactions
Copper(II) oxide dissolves in mineral acids such as hydrochloric acid, sulfuric acid or nitric acid to give the corresponding copper(II) salts:
CuO + 2 HNO3 → Cu(NO3)2 + H2O
CuO + 2 HCl → CuCl2 + H2O
CuO + H2SO4 → CuSO4 + H2O
It reacts with concentrated alkali to form the corresponding cuprate salts:
2 MOH + CuO + H2O → M2[Cu(OH)4]
It can also be reduced to copper metal using hydrogen, carbon monoxide, or carbon:
CuO + H2 → Cu + H2O
CuO + CO → Cu + CO2
2 CuO + C → 2Cu + CO2
When cupric oxide is substituted for iron oxide in thermite the resulting mixture is a low explosive, not an incendiary.
Benefits
Cupric oxide is an oxide of the mineral copper. It is an essential element needed by the body to perform a host of functions.
Cupric oxide is used by specific enzymes to help in the production of energy, to create collagen and elastin, to metabolize iron, and in many functions of the brain and central nervous system. Cupric oxide is found in health supplements such as vitamins and health aid treatments.
Copper is a mineral that is needed in the body in small doses but has the ability to become toxic at high levels. Additional supplements of copper beyond what you should get in your normal diet should be discussed with a doctor.
General Description
Copper oxides (Cu2O, CuO) are p-type semiconductor materials with small band gap energy. High physical and chemical stability of metal oxide nanoparticles renders them extremely useful in catalytic applications.The structures of the compounds are monoclinic. Nanoscaled copper oxide compounds can be prepared by thermal plasma technology. A study reports its antimicrobial properties.
Health Hazard
Exposures to copper fume cause fever, chills, muscle aches, nausea, dry throat, coughing, weakness, lassitude, irritation to the eyes, nose, throat, skin, upper respiratory tract, chest tightness, nose bleed, edema, and lung damage. Symptoms of copper fume poisoning also include metallic or sweet taste, skin itching, skin rash, skin allergy, and a greenish color to the skin, teeth, and hair. Workers have increased risk of Wilson’s disease.
Pharmacokinetics
For pharmacodynamic information of copper, refer to drug entry for Copper. Copper(II) oxide nanoparticles are known to generate reactive oxygen species (ROS), leading to cytotoxicity . In a comparative toxicity assay, nanoparticles caused significant mitochondrial depolarization leading to DNA damage . In the human skin organ culture study, topical application of copper oxide (CuO) nanoparticles induced inflammatory cytokine secretion and necrosis in vitro, indicating that the nanoparticles may adhere to the skin surface and react with the local acidic environment .
Metabolism
Cupric oxide may dissolve in acids including hydrochloric acid to form copper (II) chloride . As an inorganic compound, cupric oxide is unlikely to undergo biological degradation.
Precautions
Occupational workers should use protective clothing, such as suits, gloves, footwear, and headgear, and promptly change the contaminated clothing/work dress. Workers should not eat, smoke, or drink where copper dust or powder is handled, processed, or stored. Workers should wash hands carefully before eating, drinking, smoking, or using the toilet. The workplace should have a vacuum or a wet method facility to reduce the metal dust during cleanup
Properties of Cupric oxide
| Melting point: | 1326 °C |
| Density | 6.315 |
| refractive index | 2.63 |
| storage temp. | no restrictions. |
| solubility | Aqueous Acid (Slightly), Methanol (Slightly) |
| form | powder |
| Specific Gravity | 6.3-6.49 |
| color | Brown to black |
| Odor | at 100.00?%. odorless |
| PH | 7 (50g/l, H2O, 20℃)(slurry) |
| Water Solubility | insoluble |
| Merck | 14,2646 |
| Dielectric constant | 18.1(Ambient) |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 0.1 mg/m3; TWA 1 mg/m3 |
| Stability: | Stable. Incompatible with reducing agents, hydrogen sulfide, aluminium, alkali metals, finely powdered metals. |
| CAS DataBase Reference | 1317-38-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Copper(ii) oxide(1317-38-0) |
| EPA Substance Registry System | Cupric oxide (1317-38-0) |
Safety information for Cupric oxide
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P501:Dispose of contents/container to..… |
Computed Descriptors for Cupric oxide
Cupric oxide manufacturer
Jaiswal S Cyber Shop
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Cupric Oxide 98%View Details
Cupric Oxide 98%View Details -
 Copper(II) oxide CAS 1317-38-0View Details
Copper(II) oxide CAS 1317-38-0View Details
1317-38-0 -
 Copper(II) oxide CAS 1317-38-0View Details
Copper(II) oxide CAS 1317-38-0View Details
1317-38-0 -
 Copper(II) oxide CAS 1317-38-0View Details
Copper(II) oxide CAS 1317-38-0View Details
1317-38-0 -
 Copper(II) oxide, For ACS analysis CAS 1317-38-0View Details
Copper(II) oxide, For ACS analysis CAS 1317-38-0View Details
1317-38-0 -
 Copper(II) oxide, For ACS analysis CAS 1317-38-0View Details
Copper(II) oxide, For ACS analysis CAS 1317-38-0View Details
1317-38-0 -
 Copper(II) oxide CAS 1317-38-0View Details
Copper(II) oxide CAS 1317-38-0View Details
1317-38-0 -
 Copper(II) oxide CAS 1317-38-0View Details
Copper(II) oxide CAS 1317-38-0View Details
1317-38-0
