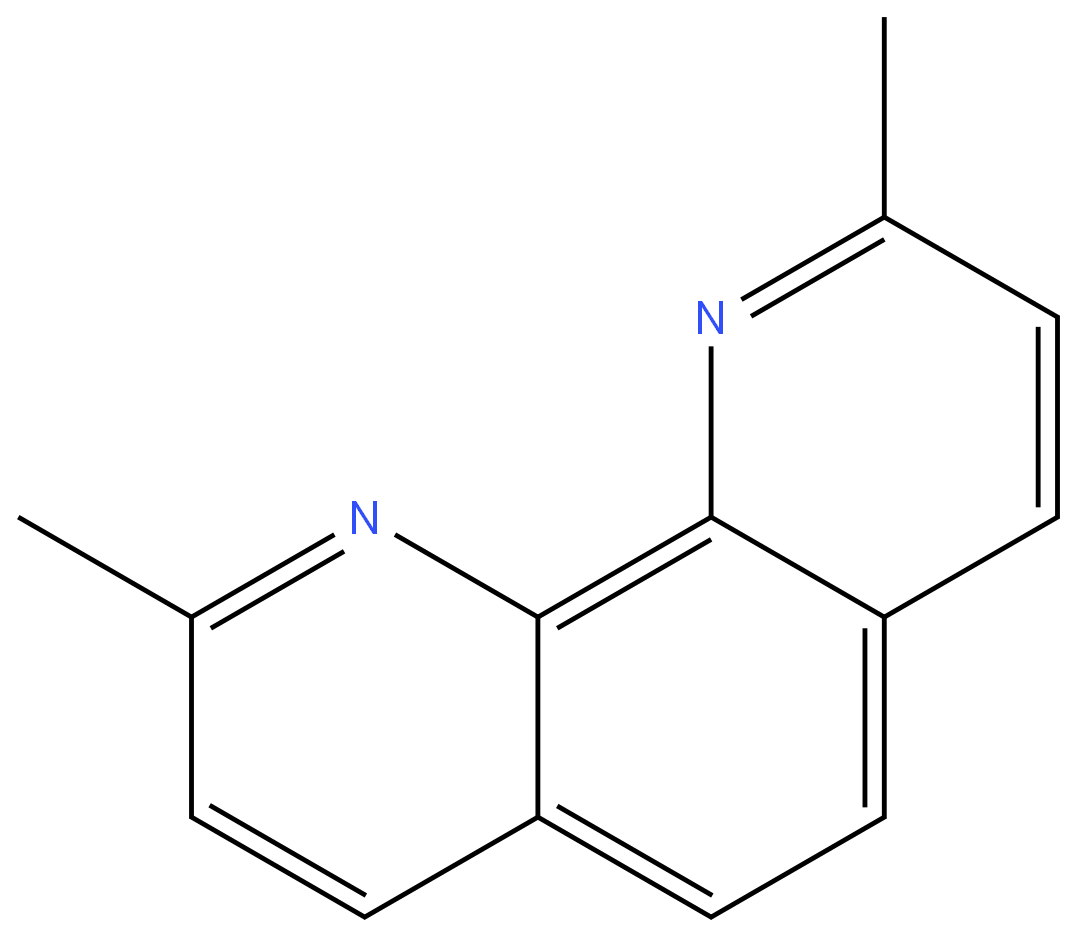
Neocuproine
Synonym(s):2,9-Dimethyl-1,10-phenanthroline;DMPHEN;Neocuproine
- CAS NO.:484-11-7
- Empirical Formula: C14H12N2
- Molecular Weight: 208.26
- MDL number: MFCD00004973
- EINECS: 207-601-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-24 14:33:23
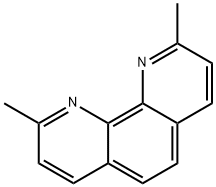
What is Neocuproine?
Chemical properties
OFF-WHITE TO VERY PALE YELLOW CRYSTALLINE POWDER
The Uses of Neocuproine
Due to the steric hindrance of the methyl groups attached to the carbon atoms
adjacent to the nitrogen donor atoms of phenanthroline, the reagent does not
give the low spin vivid red complex characteristic of phenanthroline derivatives
with iron(II). However, in the presence of reducing agents it reacts with copper to
give a copper(I) complex of composition MA2 and of tetrahedral symmetry. This
chelate is insoluble in water and can be extracted by chloroform in which the absorption
maximum of the complex appears at 457 nm. In this way the concentration
of the complex can be measured. The method is suitable for the determination
of copper in iron, manganese and vanadium ores even in the presence of
aluminium, germanium, titanium and silicon.
Neocuproine is today considered one of the most selective reagents. Unfortunately,
it is rather expensive. 2,3-bis-(2-pyridyl)quinoxaline, prepared by Belcher
et al. by condensation of o-diketone, 2,2'-dipyridyl and 0-phenylenediamine(44)
contains the functional grouping characteristic of cuproine; it is quite suitable for
the determination of copper and it is cheap. Starting with various substituted
o-phenylene-diamines Belcher synthesized 25 different quinoxaline derivatives,
of which 2,3-bis-[2-(-methyl)-pyridyl)]quinoxaline proved to be identical with
neocuproine as regards analytical selectivity. Besides copper, titanium(III) is the
only metal ion which gives a colour reaction with the reagent. However, the
coloured titanium(III) complex is formed only at lower pH and hence it does not
interfere with the determination of copper. The copper complex of the reagent can
be extracted quantitatively with isopentyl alcohol usually as a perchlorate ion pair.
The Uses of Neocuproine
Neocuproine is a phenanthroline based metal ion chelating agent.
What are the applications of Application
Neocuproine is a phenanthroline based metal ion chelating agent
Definition
ChEBI: A member of the class of phenanthrolines that is 1,10-phenanthroline bearing two methyl substituents at positions 2 and 9.
Purification Methods
Purifiy it as the hemihydrate by crystallisation from water and as the anhydrous base from *benzene. [Beilstein 23/8 V 527.]
Properties of Neocuproine
| Melting point: | 159-164 °C |
| Boiling point: | 337.46°C (rough estimate) |
| Density | 1.1345 (rough estimate) |
| RTECS | SF8380000 |
| refractive index | 1.6152 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | methanol: 0.1 g/mL, clear |
| form | crystalline |
| pka | 6.01±0.30(Predicted) |
| color | white to beige |
| Water Solubility | slightly soluble |
| Sensitive | Light Sensitive |
| Merck | 6449 |
| CAS DataBase Reference | 484-11-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,10-Phenanthroline, 2,9-dimethyl-(484-11-7) |
| EPA Substance Registry System | 1,10-Phenanthroline, 2,9-dimethyl- (484-11-7) |
Safety information for Neocuproine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Neocuproine
| InChIKey | IYRGXJIJGHOCFS-UHFFFAOYSA-N |
Neocuproine manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Neocuproine 98%View Details
Neocuproine 98%View Details -
 Neocuproine 98% (HPLC) CAS 484-11-7View Details
Neocuproine 98% (HPLC) CAS 484-11-7View Details
484-11-7 -
 Neocuproine CAS 484-11-7View Details
Neocuproine CAS 484-11-7View Details
484-11-7 -
 Neocuproine, GR 99%+ CAS 484-11-7View Details
Neocuproine, GR 99%+ CAS 484-11-7View Details
484-11-7 -
 Neocuproine CAS 484-11-7View Details
Neocuproine CAS 484-11-7View Details
484-11-7 -
 NEOCUPROINE AR CAS 484-11-7View Details
NEOCUPROINE AR CAS 484-11-7View Details
484-11-7 -
 Neocuproin (2,9-Dimethyl-1,10-Phenanthroline)View Details
Neocuproin (2,9-Dimethyl-1,10-Phenanthroline)View Details
484-11-7 -
 2,9-Dimethyl-1,10-PhenanthrolineView Details
2,9-Dimethyl-1,10-PhenanthrolineView Details
484-11-7
