nalbuphine
- CAS NO.:20594-83-6
- Empirical Formula: C21H27NO4
- Molecular Weight: 357.45
- EINECS: 243-901-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:43:59
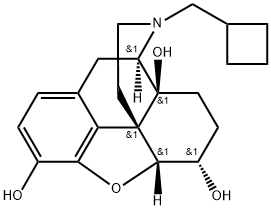
What is nalbuphine ?
Absorption
The mean absolute bioavailability was 81% and 83% for the 10 and 20 mg intramuscular doses, respectively, and 79% and 76% following 10 and 20 mg of subcutaneous nalbuphine. Clinical studies show that the duration of analgesic activity of the drug can range from 3 to 6 hours.
Toxicity
Oral, acute LD50 is 1100 mg/kg in dog. Symptoms of overdose include primarily sleepiness and mild dysphoria.
Chemical properties
Pale Yellow Solid
Originator
Nubain,Du Pont,US,1979
The Uses of nalbuphine
Nalbuphine is a mixed opioid agonist-antagonist. Nalbuphine is an analgesic (narcotic).
The Uses of nalbuphine
Mixed opioid agonist-antagonist. Analgesic (narcotic).
Background
A narcotic used as a pain medication. It appears to be an agonist at kappa opioid receptors and an antagonist or partial agonist at mu opioid receptors. Nalbuphine is the only opioid analgesic that is not a controlled substance in the United States.
Indications
For the relief of moderate to severe pain.
Definition
ChEBI: Nalbuphine is an organic heteropentacyclic compound. It has a role as a mu-opioid receptor antagonist and an opioid analgesic. It derives from a hydride of a morphinan.
Manufacturing Process
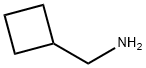
To a slurry of 110.5 g of 14-hydroxydihydronormorphinone in 2.5 liters of
methylene chloride and 280 ml of triethylamine was added a solution of 106 g
of cyclobutanecarboxylic acid chloride in 500 ml of methylene chloride. The
temperature of the reaction mixture was maintained at 20°C to 25°C during
the addition. After 5 minutes the reaction mixture was brought to reflux and
heated for 5 hours.
It was then cooled, washed with water, dried over sodium sulfate and
evaporated to dryness. The residue was crystallized from benzene and
pentane to give 138.5 g of the dicyclobutanecarbonyl derivative, melting point
about 112°C (dec.).
The dicyclobutanecarbonyl derivative (136.7 g) was dissolved in 200 ml of
tetrahydrofuran and added dropwise to a suspension of 34.2 g of lithium
aluminum hydride in 1 liters of tetrahydrofuran. The temperature of the
mixture rose to reflux during the addition. Reflux was maintained for 2 hours
after the addition was completed. After cooling, 110 ml of ethyl acetate was
added dropwise, followed by 30 ml of water, followed by a solution of 53 g of
ammonium chloride in 125 ml of water. The resulting mixture was filtered and
the inorganic precipitate was washed with methanol. Evaporation of the
combined filtrates gave 66 g of N-cyclobutylmethyl-14-hydroxydihydronormorphinone, melting point 229°C to 231°C.
brand name
Nubain (Endo).
Therapeutic Function
Analgesic
Pharmacokinetics
Nalbuphine is a synthetic opioid agonist-antagonist analgesic of the phenanthrene series. Nalbuphine's analgesic potency is essentially equivalent to that of morphine on a milligram basis. The opoioid antagonist activity of nalbuphine is about one-fourth to that of nalorphine and 10 times to that of pentazocine. Nalbuphine by itself has potent opioid antagonist activity at doses equal to or lower than its analgesic dose. When administered following or concurrent with mu agonist opioid analgesics (e.g., morphine, oxymorphone, fentanyl), nalbuphine may partially reverse or block opioid-induced respiratory depression from the mu agonist analgesic. Nalbuphine may precipitate withdrawal in patients dependent on opioid drugs. Nalbuphine should be used with caution in patients who have been receiving mu opioid analgesics on a regular basis.
Metabolism
Not Available
Properties of nalbuphine
| Melting point: | 230.5° |
| Boiling point: | 566.6±50.0 °C(Predicted) |
| Density | 1.44±0.1 g/cm3(Predicted) |
| solubility | soluble in Methanol |
| form | Solid |
| pka | 9.39±0.60(Predicted) |
| color | White |
Safety information for nalbuphine
Computed Descriptors for nalbuphine
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
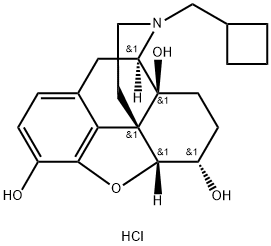

![17-[CYCLOBUTYLMETHYL]-4,5-EPOXYMORPHINAN-3,6,14-TRIOL HYDROCHLORIDE, DIHYDRATE](https://img.chemicalbook.in/CAS/GIF/59052-16-3.gif)

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4