NALBUPHINE HYDROCHLORIDE
Synonym(s):17-(Cyclobutylmethyl)-4,5-epoxymorphinan-3,6,14-triol;17-(Cyclobutylmethyl)-4,5-epoxymorphinan-3,6,14-triol hydrochloride hydrate
- CAS NO.:23277-43-2
- Empirical Formula: C21H28ClNO4
- Molecular Weight: 393.91
- MDL number: MFCD00069325
- EINECS: 245-549-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
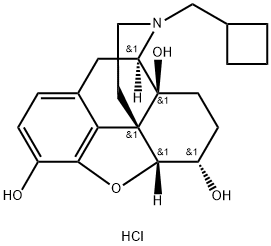
What is NALBUPHINE HYDROCHLORIDE?
Originator
Nubain,Du Pont,US,1979
The Uses of NALBUPHINE HYDROCHLORIDE
Nalbuphine is the free base form of Nalbuphine Hydrochloride. Nalbuphine is a mixed opioid agonist-antagonist. Nalbuphine is an analgesic (narcotic).
The Uses of NALBUPHINE HYDROCHLORIDE
Analgesic;Opioid ligand
Manufacturing Process
To a slurry of 110.5 g of 14-hydroxydihydronormorphinone in 2.5 liters of
methylene chloride and 280 ml of triethylamine was added a solution of 106 g
of cyclobutanecarboxylic acid chloride in 500 ml of methylene chloride. The
temperature of the reaction mixture was maintained at 20°C to 25°C during
the addition. After 5 minutes the reaction mixture was brought to reflux and
heated for 5 hours.
It was then cooled, washed with water, dried over sodium sulfate and
evaporated to dryness. The residue was crystallized from benzene and
pentane to give 138.5 g of the dicyclobutanecarbonyl derivative, melting point
about 112°C (dec.).
The dicyclobutanecarbonyl derivative (136.7 g) was dissolved in 200 ml of
tetrahydrofuran and added dropwise to a suspension of 34.2 g of lithium
aluminum hydride in 1 liters of tetrahydrofuran. The temperature of the
mixture rose to reflux during the addition. Reflux was maintained for 2 hours
after the addition was completed. After cooling, 110 ml of ethyl acetate was
added dropwise, followed by 30 ml of water, followed by a solution of 53 g of
ammonium chloride in 125 ml of water. The resulting mixture was filtered and
the inorganic precipitate was washed with methanol. Evaporation of the
combined filtrates gave 66 g of N-cyclobutylmethyl-14-hydroxydihydronormorphinone, melting point 229°C to 231°C.
brand name
Nubain (Endo).
Therapeutic Function
Analgesic
Acquired resistance
Nalbuphine is an antagonist at μ receptors and an agonist at κ receptors. As an antagonist, it has approximately one-fourth the potency of naloxone, and it produces withdrawal when given to addicts. On a weight basis, the analgesic potency of nalbuphine approaches that of morphine. An intramuscular injection of 10 mg will give about the same degree and duration of analgesia as an equivalent dose of morphine.
Clinical Use
Nalbuphine is only available for parenteral dosage. Its elimination half-life is 2 to 3 hours. Metabolism of nalbuphine is by conjugation of the 3-OH group, and greater than 90% of the drug is excreted as conjugates in the feces.
Side Effects
Side effects of nalbuphine are like those of other κ. Dysphoria is not as common as with pentazocine. Sedation is the most common side effect. Nalbuphine does not have the adverse cardiovascular properties found with pentazocine and butorphanol. Nalbuphine has low abuse potential and is not listed under the Controlled Substances Act.
Properties of NALBUPHINE HYDROCHLORIDE
| Flash point: | 9℃ |
| storage temp. | -20°C |
| solubility | H2O: soluble |
Safety information for NALBUPHINE HYDROCHLORIDE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P262:Do not get in eyes, on skin, or on clothing. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for NALBUPHINE HYDROCHLORIDE
NALBUPHINE HYDROCHLORIDE manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Nalbuphine hydrochloride hydrate CAS 23277-43-2View Details
Nalbuphine hydrochloride hydrate CAS 23277-43-2View Details
23277-43-2 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
