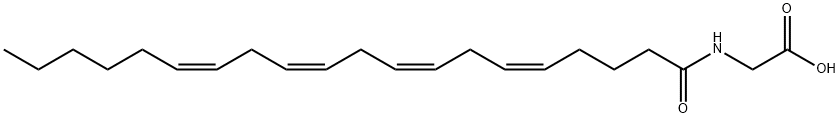
NAGLY
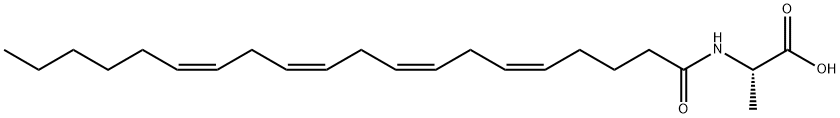
Synonym(s):methylene chloride solution;N-[(5Z,8Z,11Z,14Z)-1-oxo-5,8,11,14-eicosatetraen-1-yl]-glycine
- CAS NO.:179113-91-8
- Empirical Formula: C22H35NO3
- Molecular Weight: 361.52
- MDL number: MFCD03791313
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
What is NAGLY?
Description
Arachidonoyl glycine (N-
The Uses of NAGLY
Arachidonoyl glycine (N-arachidonyl glycine; NAGly) has been isolated from cell cultures treated with arachidonoyl ethanolamide (AEA; anandamide), from extracts of mammalian brain, and has also been synthesized as an analog of AEA for structure/activity testing. NAGly may be produced endogenously via oxidation of AEA, or by transacylation of arachidonoyl CoA. NAGly is reported to have analgesic activities in whole animal experiments. Since it seems to be a very poor ligand for the CB1 receptor, these effects are probably mediated via other signaling pathways.[Cayman Chemical]
The Uses of NAGLY
An endogenous anandamide-like compound; also acts as a T-type Ca2+ channel blocker and an endogenous GLYT2 inhibitor.
What are the applications of Application
N-Arachidonoyl glycine (solution in ethanol) is a compound that lacks affinity for CB1 receptors but inhibits FAAH
What are the applications of Application
N-Arachidonoyl glycine is an endogenous anandamide-like compound
Definition
ChEBI: N-arachidonoylglycine is biologically active derivative of anandamide It is a N-acylglycine and a fatty amide. It is functionally related to an arachidonic acid. It is a conjugate acid of a N-arachidonoylglycinate.
Biological Activity
Endogenous anandamide-like compound. Lacks affinity for CB 1 receptors (K i > 10 μ M), VR1 receptors (EC 50 > 10 μ M) and anandamide transporters (IC 50 > 50 μ M) but causes hot-plate analgesia in mice when given orally, and suppresses tonic inflammatory pain. Also endogenous GlyT2 inhibitor.
Storage
-20°C (desiccate)
References
1) Huang et al. (2001), Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain; J. Biol. Chem., 276 42639 2) McHugh et al. (2012), Δ(9)-Tetrahydrocannabinol and N-arachidonylglycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells; Br. J. Pharmacol., 165 2414 3) McHugh et al. (2012), siRNA knockdown of GPR18 receptors in BV-2 microglia attenuates N-arachidonyl glycine-induced cell migration; J. Mol. Signal., 7 10 4) Takenouchi et al. (2012), N-arachidonyl glycine induces macrophage apoptosis via GPR18; Biochem. Biophys. Res. Commun., 418 366 5) Burstein et al. (2011), Resolution of inflammation by N-arachidonoylglycine; J. Cell Biochem., 112 3227 6) Console-Bram et al. (2017), N-arachidonoyl glycine, another endogenous agonist of GPR55; Biochem. Biophys. Res. Commun., 490 1389 [FOCUS CITATION]
Properties of NAGLY
| Boiling point: | 560.9±50.0 °C(Predicted) |
| Density | 0.985±0.06 g/cm3(Predicted) |
| storage temp. | −20°C |
| solubility | DMSO: >5 mg/mL, soluble |
| form | waxy solid |
| pka | 3.58±0.10(Predicted) |
| color | off-white |
| Sensitive | Air Sensitive |
| Stability: | Stable for 2 years from date of purchase as supplied. Product is subject to oxidation. Solutions in degassed DMSO may be stored at -20°C for up to 1 month. |
Safety information for NAGLY
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for NAGLY
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 N-arachidonoylglycine CAS 179113-91-8View Details
N-arachidonoylglycine CAS 179113-91-8View Details
179113-91-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
